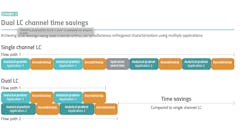
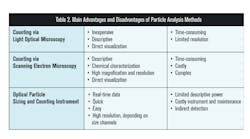
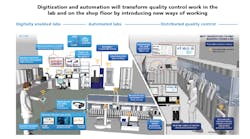
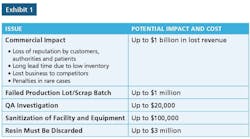
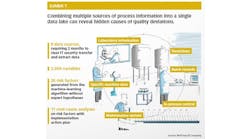
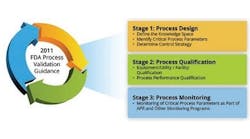
QRM Process
Quality risk management (QRM) is a systematic process that assesses risk to the quality of a drug product. Here you will find broad pieces about strategic operational philosophies or risk management plans geared towards controlling risk or regulating quality in manufacturing.