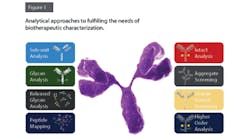
QbD
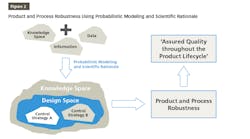
Quality by design (QbD) involves a science-based approach for developing drugs that is highly supported by the FDA. A robust QbD process requires an intimate understanding of the product characteristics and manufacturing process so that manufacturers can understand which aspects of the product/process are critical and which can be changed with minimal impact.