The life sciences global supply chain has evolved over the last three decades as much of the U.S. domestic production of pharmaceuticals, medical devices, and equipment transitioned overseas in favor of lower labor and overhead costs.
According to the U.S. Food and Drug Administration, 88% of active pharmaceutical ingredient (API) manufacturing has shifted offshore over the past decade alone. In the last year, however, the pandemic has highlighted several weak links in the current global supply chain and heightened concerns about national security and public safety from both sides of the political spectrum.
Now, with the push to move production back to the U.S., pharma companies may need to rethink their procurement processes and sourcing strategies to enable their reshoring efforts and compete for the future. And legislative incentives and penalties may further drive the need to pursue onshore supply.
Challenges to reshoring
In his March 2021 American Jobs Plan, President Biden called on Congress to invest $300 billion to retool and revitalize American manufacturers.
This plan includes:
- $50 billion to strengthen manufacturing supply chains for critical goods
- $30 billion over four years to protect Americans from future pandemics, including shoring up our nation’s strategic national stockpile (SNS) and onshoring APIs
- $52 billion in domestic manufacturer investments to promote innovation, adaptation, and scale to win the industries of the future, including life sciences
Further, President Biden’s April 2021 Made in America Tax Plan proposes a number of tax policy amendments that could trigger changes to the supply chain. The proposed changes include a global minimum tax and potential disallowance of tax deductions for payments to foreign entities that are not taxed at the minimum tax rate.
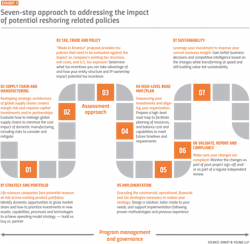
Reversing operations established over decades can require a coordinated, structured, and multi-pronged approach, including a holistic assessment of the company’s strategy and portfolio, supply chain and manufacturing, and tax, trade, and policy considerations as illustrated in steps 1–3 in Exhibit 1.
Based on the outcome of the assessment, pharma executives can take action to revamp their procurement process.
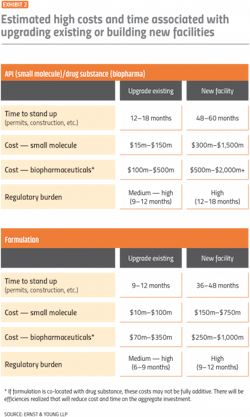
Reshoring is likely to come with large costs and take several years as companies upgrade existing facilities or build new ones.
Developing a high-level road map can underscore the investments required in building or upgrading capacity. Per Exhibit 2, the cost of building a new biopharmaceutical facility can top $2 billion and take five years to construct, not including up to 18 months to secure the necessary regulatory approvals.
For this reason, procurement can play a critical role in enabling reshoring efforts.
For example, if the assessment results in the decision to reshore while avoiding capital investments by seeking domestic and regional partnerships, procurement can help create supply chain resiliency as well as ensure the sustainability of the benefits from reshoring by identifying the right partners, establishing relationships, and securing contracts.
Strategic partnerships
An alternate approach to constructing new or upgrading existing facilities while potentially accelerating implementation of reshoring is for life science companies to better leverage contract manufacturing organizations (CMOs). Several leading CMOs have each invested or announced plans to invest $10–$90 million in expanding some of their U.S. manufacturing facilities. Others have more than doubled their U.S. manufacturing capacity within the past three years.
To establish an optimal pool of strategic partners, procurement will not only need to assess CMOs’ capabilities, capacities, and manufacturing footprints but also evaluate how new technologies can best be utilized to enhance manufacturing efficiencies. Procurement may be critical in not just negotiating supply contracts but also in licensing contracts with new technology vendors that could potentially accelerate onshoring efforts and help reduce costs.
Using a purchasing consortium to facilitate reshoring
Establishing a procurement-related consortium that links life science companies with key ecosystem partners, such as CMOs, contract packaging organizations and third-party logistics providers could further help drive sourcing and manufacturing efficiencies. A consortium consists of two or more independent organizations that join to collaboratively increase buying power and leverage economies of scale on materials, services, and solutions offered by vendors.
It can be set up by a third-party manager that aggregates volume across members or established directly by a life sciences company as the sponsor before being pitched to other potential members.
Based on EY experience and public sources, the top benefits of consortiums are risk-sharing, accelerated learning, and cost savings. In the medical industry, 75% of all medical and surgical supply dollars and 82% of pharmaceutical supply expenditures are already negotiated through influential purchasing consortiums.
Consortiums across a broad array of industries and applications have proven to be a very successful model for attaining procurement efficiencies and improving the security of supply.
Incentivizing SNS contractual obligations
The pandemic highlighted the need to build up the strategic national stockpile of essential medicines, sparking a bi-partisan push to make sure the SNS is well stocked for future emergencies.
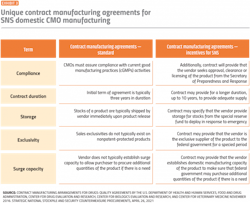
Given CMO margin pressures coupled with the uncertainty of essential medicine volume requirements, CMOs may deprioritize capacity. Due to the criticality of SNS essential medicines, robust and differentiating contract manufacturing agreements can further incentivize, enhance partnerships and reduce risk with CMOs.
Procurement plays an essential role in thoughtfully setting up these CMO contracts to meet domestic manufacturing requirements and secure proper fulfillment to satisfy SNS obligations (Exhibit 3).
Recommendations
With the high costs and time associated with setting up existing and new manufacturing facilities, procurement can play a more integral role in solidifying reshoring efforts.
By forming strategic partnerships, leveraging purchasing consortiums, and establishing robust contract manufacturing agreements, pharma companies can be better equipped to minimize the risks of national security threats, and optimize supply chain resiliency and costs.