The Third Way for ICH Q7A: A Proposal for Updating Guidance for API Inspections
Editor’s Note: This is another installment in a series of articles on the need to update ICH guidances. For the first article in the series, Ciurczak’s analysis of Q2 (R1), click here.
The ICH Q7A Guidance was intended as a showpiece for multi-jurisdictional cooperation in establishing methodology and standards for API inspections. It was the first internationally harmonized tripartite GMP guidance developed jointly by industry and regulators under the ICH umbrella. It establishes one global GMP standard for APIs and is intended to provide mutual recognition of GMPs in API production. (Note: Q7A does not apply to steps prior to the introduction of the defined API starting material.)
However, in light of recent problems with outsourced APIs and the growing number of small pharma houses, the ability of ICH Guidances to protect consumers is under question. The 2001 document assumes use of cGMPs by manufacturers, primarily in the signatory countries.
|
Definition of “API” From the intended use clause: “Any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that, when used in the production of a drug, becomes an active ingredient of the drug product.” From the pharmacological activity clause: “Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure and function of the body.” From the FD&C Act: “Section 501(a)(2)(B) of the Act requires that all drugs be manufactured, processed, packed, and held in accordance with current good manufacturing practice.” [Note: CGMP regulations (21 CFR 210 and 211) apply only to preparation of drug products. These CGMP regulations apply to finished dosage form drugs (under CFR 210.3(b)(4) and 211.1) and are not binding requirements for chemical manufacturing.] |
The current Q7A Guidance was approved in August of 2001, several months before the U.S. FDA’s subcommittee on PAT was even convened by Ajaz Hussain of CDER/FDA. That alone indicates that PAT was not a concern of the ICH committee working out the details of this Guidance. There is no mention of process measurements in the Guidance, only GMPs, which, according to most QA personnel, do not cover PAT/QbD directly.
The guidance was intended to facilitate API inspections by supplying uniform guidelines to inspectors in all participating countries. It impacts any manufacturer that markets APIs in ICH regions and addresses the uniqueness of API processes (that is, drug “products” are physical mixtures, while APIs are the result of chemical synthesis).
The suggestions and methodologies in this Guidance are based on:
- laboratory analyses of, usually, a statistical sample of the API lot; or
- the Certificate of Analysis from the manufacturer.
Both approaches are based on experience with “Western” suppliers in the U.S., Europe, Canada, or Japan. Since the legal systems within these countries had jurisdiction over the supplier, there was little illegality involved. In-house tests, used by QC, are mainly to identify the product possibly caused by mislabeling problems. These confirm the grade (polymorphic form, particle size, hydrous or anhydrous) and purity (testing for potential break-down products—e.g., % salicylic acid in aspirin). They were not designed to detect deliberate fraud: e.g., melamine in gluten or sulfated chondroitin in heparin.
Adulterants were apparently chosen to fool the tests run on the materials mentioned. For instance, melamine polymer was used by some agro-businesses a quarter century or so ago to fool the Kjeldahl nitrogen test, making the protein content seem higher, garnering more profit. The melamine monomer, quite toxic, was added to the milk in China to cover up the watering-down of the product, covering up fraud. Companies using simple, chemical spot tests (in compendia such as EP, USP, and ASTM) did so under the assumption of a scrupulous supplier with a possibility of “known” and reasonable contaminants, well documented in the normal production of a material. They were designed for safety and potential breakdown of the API, not chemicals deliberately added to fool them.
The diligence of the FDA and USP in the heparin scandal is noteworthy. The speed with which the contaminants were identified and new procedures instituted is remarkable and should be congratulated as well as noted. However, considering the effort of so many researchers and the costs involved, can this be done for all imported APIs, raw materials, finished drug products, and intermediates every batch ad infinitum? And, if this was to become protocol, what effects will it have on the industry and cost to consumers?
If a material is imported to save 30-40% of its cost, but several weeks of testing, using sophisticated instrumentation, are needed to pass the imported lots, what will happen to the final cost of goods? QC departments will have enormous pressure to expand and add equipment not usually associated with routine analyses: capillary electrophoresis, multi-dimensional NMR and numerous other techniques. [For further details, see the “Tools” section below.]
The time involved will diminish the profit margin and/or increase the cost of goods to the patients. Smaller, independent generic Pharma companies do not routinely have the ability to perform 2-D NMR and capillary electrophoresis; in fact, few larger firms supply their QC departments with such equipment, either. The cost of either purchasing the equipment (plus hiring people to run it) or paying someone else to run the tests would be quite a hardship. I can envision a number of small houses closing in short order.
The Light at the End of the Tunnel
There is a third approach, however. The only two choices are not merely “old time” wet tests or super-sophisticated science-based tests. A combination of on-site qualification and some of the equipment used in PAT can be used to ensure raw material purity. With a new Congress, willing to spend money to help its citizens, this may be the time to give the FDA all the tools it needs to inspect overseas sites. Pharmaceutical companies are “contracting,” “re-sizing,”, or just plain “downsizing” at the moment. While many positions being eliminated are in sales and marketing, a large number of very good scientists are also being cashiered, too.
If FDA adds to its force of inspectors, they will need back-up in the lab as well. Should Congress be persuaded to fully fund FDA’s mandate to biennial inspections, these talented scientists, already familiar with GMPs, could help fill the ranks of (quite talented) existing scientists at FDA. With enough inspectors to “coach” overseas vendors to learn and follow cGMPs, the “difficulties” of questionable APIs could be manageable. And, as far as “on-site” tools . . .
In fact, hasn’t NIR (and now Raman) been suggested by FDA for years? NIR has been used for raw materials since 1985 and, according to Dr. Hussain, is “too obvious to even mention in the Guidance.” The simplistic answer is NOT to do super-scientific assays on every milligram of every API produced in the world. The sensible approach would be to set up screening of raw materials (excipients as well as APIs) using spectrometric methods. NIR and Raman have proven excellent choices for decades.
The slightest anomaly in the screening process could trigger the use of more sophisticated methods. However, even if up to 10-20% of the samples were sent for second level tests, it would still greatly simplify the process . . . not to mention alleviate the staggering cost to medium and small-sized companies.
Many of the companies facing the problem already have NIR capability, so why not extend it to overseas API screening? As an extra benefit, many properties of APIs and excipients would also be cataloged. Under QbD, the physical properties are as important as the chemical. It is likely that companies will be doing spectral screening someday for particle size, crushability, porosity, etc., anyway. So why not combine the work and just add APIs to the list. It will speed up the work and save a few billion dollars or euros along the way.
In the long run, this multi-disciplined approach will both ensure the safety of our drug supply and keep the cost of goods sold (COGS) reasonable.
Tools Used in the Assay of APIs
The heparin study (problem) brought several interesting techniques into the “popular” scientific press: namely two-dimensional NMR (Nuclear Magnetic Resonance) and CE (Capillary Electrophoresis). Now, most analytical chemists know what these techniques are, but they are somewhat unusual for routine analyses.
NMR (normal or one-dimensional) is taught in virtually every undergraduate Instrumental Analysis course. Students use tables and such to analyze simple molecules such as alcohols and amines. When a complex biological molecule, like heparin, for instance, is scanned by a “typical” NMR, it is beyond most chemists’ ability to interpret the spectra. With computer-aided instruments this challenge becomes doable.
Figure 1. “Contour map” of frequencies versus intensity of a two-dimensional NMR scan.
In “normal,” one-dimensional NMR, intensity is plotted versus frequency, giving us what is recognized as an “NMR spectrum.” In two-dimensional spectroscopy, intensity is plotted as a function of two frequencies, usually called F1 and F2. There are various ways of representing such a spectrum on paper, but the one most usually used is to make a contour plot in which the intensity of the peaks is represented by contour lines drawn at suitable intervals, in the same way as a topographical map (see Figure 1).
The position of each peak is specified by two frequency coordinates corresponding to F1 and F2. Two-dimensional NMR spectra are always arranged so that the F2 coordinates of the peaks correspond to those found in the normal one-dimensional spectrum, and this relation is often emphasized by plotting the one-dimensional spectrum alongside the F2 axis (see Figure 2). This manner of representation clearly shows the presence of two components.
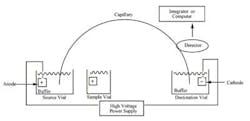
The other technique mentioned is CE. In this technique, as opposed to “regular” chromatography or electrophoresis, a narrow bore tube is used to separate charged moieties by their mass/charge ratio.
The schematic of a CE instrument is seen in Figure 3. The tube connects two buffer reservoirs charged as + and -. The samples are introduced via the sample vial which takes the place of the anode, causing sample molecules to migrate to the cathode.
While similar to gel electrophoresis, this technique allows multiple samples and the use of UV, IR, and other detection methods in lieu of simple stains, etc. The concept is simple, but cleanliness and attention to detail is critical, since such small amounts of material are being assayed . . . not to mention electricity is in play. Figure 4 shows a graphic representation of a column cross-section. In this case, silica lines the column. The electroosmotic flow is seen from anode to cathode.
Figure 3. Schematic of capillary electrophoresis set-up. While not
overly complex, it is not designed as “simple” quality control tool.
The operator needs fair amount of training on the equipment.
Figure 4. Graphic representation of column for
capillary electrophoresis: mode of operation.
It should be apparent that both 2-D NMR and CE would be quite a bit more difficult to operate and cost significantly more than standard “wet” methods seen in the USP, etc. For a simple comparison, the common techniques of HPLC and NIR are compared. The main difference here is that HPLC, like both previously mentioned techniques, requires major sample preparation. NIR uses “neat” samples while all the others require solvent extraction and dilutions, not to mention prepared standards run side-by-side.
The operating costs of HPLC versus NIR can be seen in Figure 5. The labor cost is based on $125 (USD) per hour for a trained technician (probably more, in reality). The cost of purchasing and disposing organic solvents, columns, glassware (purchase and cleaning), standards, sample vials, notebook time, and supervisor oversight are taken into account.
Both instruments are virtually alike the first year, as that includes purchasing the equipment and a NIR is usually more expensive than an HPLC. However, after the initial year, the costs for the NIR are quite obviously far less than the continuing costs to run and maintain an HPLC unit.
Figure 5: Relative cost of operating an HPLC versus a NIR (in a lab) for routine analyses of API. Similar comparisons are made for dosage form analyses as well. NIR requires no solvents, sample prep, etc., while HPLC costs continue to rise due to cost of solvents and legal disposal thereof.