Advances in the use of biological structures and processes to target human disease have resulted in the development of a broad range of innovative biotherapeutics, spanning recombinant protein-based products such as monoclonal antibodies (mAbs), cytokines and enzymes, through to drug molecules generated by microbial fermentation. The specific technologies and processes used to manufacture each of these biopharmaceutical products are diverse and specialized. However, they are all highly sophisticated and must be carefully optimized to deliver the intended results, as small changes in manufacturing conditions can have a significant impact on the quality and consistency of the product.
Consequently, to ensure the release of safe and effective products, advanced analytical methods must be employed to closely monitor the critical quality attributes (CQAs) for every batch.
Due to the way in which these drugs are manufactured, the impurities that are present often have very similar characteristics to the active ingredient itself. This can make the identification and quantification of impurities highly challenging, especially using conventional analytical techniques. Fortunately, advances in the analytical technologies used for routine workflows are helping to support the efficient manufacture and release of high-quality biotherapeutics in line with regulatory requirements. Here, we highlight how advances in biopharmaceutical characterization technologies are helping manufacturers efficiently and effectively monitor CQAs and deliver safe, high-quality medicines to patients.
More consistent characterization
mAbs are an increasingly important biotherapeutic product class that have grown steadily in use over the past three decades. Like other types of recombinant protein-based therapeutics, mAbs are large and highly complex molecules that are manufactured using sophisticated production and purification processes. However, this complexity necessitates robust and reliable characterization techniques to screen for a broad range of impurities and post-translational modifications (PTMs) introduced during manufacturing steps.
Peptide mapping is a well-established method for the characterization of mAbs and other recombinant protein-based drugs, providing primary sequence confirmation and enabling the identification and quantification of PTMs such as deamidations, glycosylations and oxidations. The technique involves enzymatically or chemically digesting mAbs to generate peptide fragments that are separated and analyzed by liquid chromatography-mass spectrometry (LC-MS) techniques.
The enzyme of choice within peptide mapping digestion workflows is trypsin. However, trypsin-based approaches are often associated with poor reproducibility, limiting confidence in results. Studies have shown that small changes in digestion conditions can result in significant variations in the number and type of PTMs, with high buffer pH and long digestion times resulting in greater concentrations of deaminated peptides.1 Additionally, trypsin-based digestion protocols typically require time-consuming manual sample handling steps that cannot be easily automated, further increasing the potential for inconsistencies in results.
Improvements in automated trypsin-based protein digestion protocols are helping to overcome these challenges by streamlining peptide mapping workflows and improving the consistency of results. The advent of commercial trypsin digest kits that include thermally stable, immobilized trypsin has enabled high-temperature protein denaturation workflows that do not require the addition of denaturants. This has resulted in faster and more reproducible protein digestion workflows that reduce post-translational modifications and deliver more consistent results. Some trypsin digest kits also enable automated sample preparation and digestion workflows, accelerating the delivery of consistent characterization data by simplifying or even eliminating error-prone manual steps. As such, these workflow innovations are helping to boost throughput and efficiency while also increasing confidence in results.
Improving throughput
Another bottleneck for manufacturers operating peptide mapping workflows is LC-MS analysis. Despite the breadth and depth of information that can be obtained from LC-MS workflows, these methods typically require lengthy column reconditioning or washing steps to re-equilibrate the column for the next sample. These steps are important to remove residues that may remain on the column, ensure stable backpressures and achieve consistent and reliable separation performance, especially if mobile phases contain buffers or ion-pair reagents. Although individual reconditioning steps may only take a matter of minutes, over the course of a typical peptide mapping sequence they can extend workflows by hours, during which time the MS instrument stands idle.
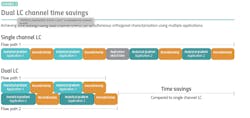
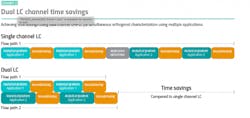
Modern dual channel ultra-high performance liquid chromatography (UHPLC) systems are helping manufacturers of biopharmaceutical products apply their resources more efficiently. These innovative systems are highly flexible and enable the use of a second channel that is supported by a separate pump and detector system. The two channels can be configured according to the needs of the application, enabling either tandem LC or LC-MS applications, or alternatively, simultaneous orthogonal characterization depending on the system set-up.
When configured to operate in tandem LC-MS mode, dual channel UHPLC systems allow the same validated method to be run across two pumps, saving considerable time compared to a single channel system (Exhibit 1). This set-up allows one channel to be used to collect data, while the second is simultaneously used to condition the column for the next analytical run. These highly efficient workflows even allow analysts to implement additional washing steps without extending total run times, helping to improve the quality of peptide mapping data obtained, without adding additional time to analytical runs.
Boosting analytical efficiency
Other characterization workflows are widely used for the analysis of mAbs and other recombinant protein-based products. Commonly used techniques include strong cation exchange (SCX) chromatography, size exclusion chromatography (SEC), and reversed-phase chromatography. Each technique depends on specific chromatography column chemistries and gradient methods, allowing manufacturers to monitor a comprehensive range of product CQAs.
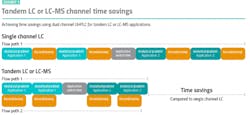
Despite the excellent analytical performance offered by the latest UHPLC systems, meeting the high throughput demands of routine biotherapeutic characterization workflows remains challenging. To perform the full range of characterization studies required to support regulatory guidelines, manufacturers must often perform separate analytical runs sequentially, setting up each new method after the previous workflow has been completed. This necessarily involves time-consuming application switching steps, extending the total analysis time required (Exhibit 2). One way for biotherapeutics manufacturers to improve throughput and capacity is to increase the number of instruments they use for characterization workflows. However, for many organizations, limited budgets and laboratory space mean that investing in additional equipment is not feasible.
The latest dual channel UHPLC systems are helping biopharma manufacturers obtain the reliable characterization results they need, using the same resources. When configured to support simultaneous orthogonal characterization, separate characterization techniques can be used to analyze the same sample, increasing the quantity of information that is obtained while improving productivity and cost per sample. By reducing sample preparation requirements and eliminating the need to change validated experimental conditions, dual channel UHPLC systems offer time and cost savings compared to single channel instrument set-ups.
Safeguarding quality and consistency
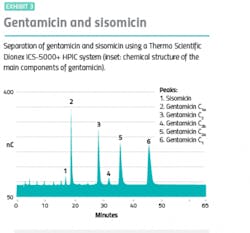
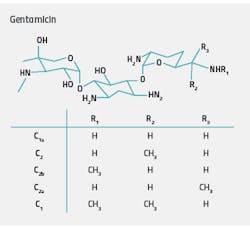
Although many of the largest categories of biotherapeutics are recombinant proteins, these are by no means the only biopharma products that require robust characterization workflows to ensure therapeutic safety and efficacy. Broad-spectrum aminoglycoside antibiotics are manufactured by bacterial fermentation processes and typically have a relatively narrow therapeutic range.2 The aminoglycoside antibiotic gentamicin, for example, is used for the treatment of serious infections caused by gram-negative bacteria. However, its application is limited due to the potential for renal and otovestibular toxicity. The small difference between the effective and toxic concentration means careful therapeutic monitoring of aminoglycoside levels is necessary to minimize the risk of adverse effects, particularly in patients with renal failure.
Ion-pairing reversed-phase liquid chromatography combined with pulsed amperometric detection (PAD) is widely used for the analysis of aminoglycoside antibiotics and related impurities. However, the number and structural similarity of the impurities that are present makes characterization challenging if high-resolution chromatographic separation is not maintained. With routine high-throughput laboratories under pressure to produce accurate results while maximizing throughput and operational efficiency, fast, robust, and reliable analytical solutions are required.
Fortunately, improvements in modern high-performance ion chromatography (HPIC) systems are helping to deliver fast, sensitive analyses without compromising on resolution. The advanced high-pressure capabilities of the latest HPIC systems enable excellentFurthermore, the availability of dedicated fast ion chromatography columns designed for rapid analysis is also boosting workflow productivity by reducing the time taken to deliver results. When these analyses are run using dual channel HPIC systems, throughput can be optimized further, cutting through routine characterization workflow bottlenecks and helping manufacturers of biotherapeutics achieve more using the same resources.
Consistent and reliable
Today’s sophisticated biopharma production workflows demand the use of robust and reliable analytical methods to monitor product CQAs and ensure the release of safe, high-quality batches. In addition, with manufacturers under sustained pressure to deliver confident results cost-effectively, the technologies used within routine biopharmaceutical characterization workflows must support efficient, high-throughput analyses. Fortunately, the latest advances in technology are helping to overcome workflow bottlenecks, helping pharma companies collect consistent and reliable biopharmaceutical characterization data faster and more efficiently.
References
1. Ren D, Pipes GD, Liu D, et al. (2009) An improved trypsin digestion method minimizes digestion-induced modifications on proteins. Analytical Biochem.; 392(1): 12–21.
2. Begg, E.J., Barclay, M.L. and Kirkpatrick C.J.M. (1999) The therapeutic monitoring of antimicrobial agents. Br. J. Clin. Pharmacol.; 47(1): 23–30.