Time and Cost Identified as Key Roadblocks to PAT and QbD; Experts Disagree
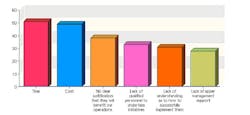
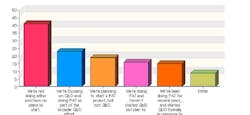
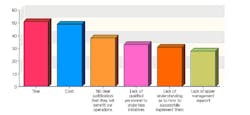
This year, 102 industry professionals responded to Pharmaceutical Manufacturing’s Process Automation and Control survey (to be analyzed in September’s issue). Nearly 35% of them said that their organizations were not implementing process analytical technologies (PAT) or Quality by Design (QbD), and had no plans to start.
43% saw time as the chief obstacle to PAT implementation, 41% cited cost, and 38% said they saw no clear justification for implementing either concept.
Last month, in a webcast presented by Pharmaceutical Manufacturing.com and sponsored by Waters Corp., a number of PAT and QbD experts examined some of the factors that appear to be holding back industry’s adoption of PAT and QbD.
Here is some of what they had to say. (For a detailed report, please visit pharmaqbd.com. To view the archived webcast discussion, which also included contributing editor and advisor Emil Ciurczak, Gawayne Mahboubian-Jones of Philip Morris International, and advisor Girish Malhotra, click here).
Ali Afnan
“…Initiatives at FDA began due to a desire for change in our industry...Quality is an ongoing demand. We have a more unforgiving global consumer base, courtesy of electronic data and news outlets, which allow the public to react very quickly to any issues. One major company that recently had quality problems saw its sales for analgesics drop by something on the order of 40-60%.
So business profitability is one driver for these initiatives.
Supply chain security is another huge business driver. Recalls define our industry and define firms, and the more recalls it has, the less successful a firm will be. And, if you look at the FDA web pages, you see lists of drug shortages, which come up on a regular basis.
Quality assurance and control have suffered a little bit because we use surrogates to define product quality. The problem is that the various definitions of quality that we use are all subjective. For instance, hardness is an attribute that we use to define quality. The patient doesn’t even appreciate that there is a test done called hardness or dissolution. Furthermore if you look at a test like dissolution, how meaningful is a relative standard deviation of 6 percent? Is it useful to have an assay STD of plus or minus 10%?
Why is it that we are accepting of products that are critical to our health and wellbeing having a STD range of plus or minus 10%?
But these specs are all end-product focused, they are not indicative of process performance and they do not allow for process control
Doing a dissolution test is like doing a post mortem on your process, which is perhaps too little and too late…”
Pedro Hernandez Abad
“…There is a fear of change and loss of control. When quality moves from lab to the manufacturing line, there’s a learning curve, and of course, there are issues of cost, and fear of the unknown, so there’s a big cultural component to implementing QbD that needs to be addressed in the transformation.
There has been a lot of discussion about mandates and incentives. The problem with those, which stems mainly from the industry’s global nature, is that the Agency can only provide a target or a goal, not a map. There are too many specific processes and particular products, and there is so much variability in terms of unit processes and products, that efforts must be left to each organizations.
One approach might be to do some benchmarking studies using “success stories” from pilot programs, but the secretive nature of the industry has made such data sharing difficult. This approach could be dangerous too, because we could create silos for different groups within the same company, for different companies and the same group of inspectors and then end up with slightly different requirements for different companies, within the same company, or within the same regulatory agency, if we’re not all on the same page.
There’s a need for champions. QbD and PAT cannot be done part time. You need to have champions who can drive the effort... The big question is ‘How much is it going to cost?’
The roadmap issue is very specific to the process. If I put $500,000 into equipment and training, I need the money back by the end of the year. An exact number is not always possible to predict. More and more companies are moving toward doing real financial viability studies for PAT. In the current environment that’s understandable.
Then there’s the lack of understanding, the question of “What’s in it for me?” But if you don’t put product quality as the object of your organization, and think of good product quality and good patient outcomes as the target, there’s no financial argument that can be made. You have to first believe in these goals and then manage risk wisely, invest properly and innovate.
Part of the problem is that we’ve stopped doing innovation. We validate, and then the learning stops there. After that, we just put out fires. There has to be a real change in the way we think….and an understanding that quality means continual improvement.
For some organizations, it’s a question of the support systems. It’s not just an instrument on the line, but the issues such as IT infrastructure and meaningful sampling strategies. Then there’s the question of which metrics will be most meaningful”
Sam Venugopal
“…The biggest barrier is concerns about return on investment. A lot of folks are trying to push this initiative through their organizations. They often stress the idea of regulatory relief and real time release. But it can require some significant investments to get to that point.
What companies have found works is taking things in smaller chunks, and asking, “How can we achieve QbD in incremental steps? Can we look at how we are capturing information within our organization, and, at a minimum, understand our processes better….and maybe get to regulatory filing faster?