Embracing single-use innovations in a post-COVID era
The COVID-19 pandemic highlighted an important turning point for the adoption of single-use systems (SUS) in biopharmaceutical production. The need for large-scale production of vaccines and therapeutics created extreme pressures to respond quickly and responsibly.
Many biomanufacturers responded to the global threat by expanding capacities with SUS technologies. The pandemic rapidly accelerated the recognition that commercial scale production could be done faster, and possibly cheaper, than with stainless steel equipment.
Pre-COVID, SUS advances were generally limited to operations at scales beyond 2,000 liters; today, 87% of manufacturing facilities are incorporating SUS technologies. By examining nearly two decades of data, the BioPlan Associates 21st Annual Report and Survey of Biopharmaceutical Manufacturing has identified the top single-use technologies that have become integral to biomanufacturing since 2006.
SUS are involved in a wide range of processes, both upstream and down. It has been well documented that SUS offers advantages including flexibility, reduced risk of cross-contamination and lower initial capital investment.
Frequently, these systems are integrated into larger, ‘hybrid’ manufacturing strategies that combine stainless steel and SUS technologies, offering a balance of scalability and flexibility.
While market adoption of SUS doubled within the first few years, adoption is leveling off after this initial market penetration. Although concerns about cost, breakage and scalability remain in the minds of bioprocessing professionals, SUS industry suppliers have continued to innovate, making SUS a mainstream platform.
Scaling adoption plateaus
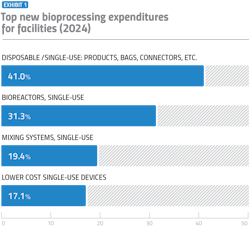
The biopharma industry has experienced a transformative shift towards the rapid adoption of single-use products and devices over the past two decades. Looking at the new expenditures focus areas cited by 2024 respondents in BioPlan’s report, we see that single-use bioreactors will maintain their profitability amongst other SUS in facilities (Exhibit 1).
The category with the largest investment has been single-use products, which have become increasingly popular. Single-use bioreactors, a pivotal innovation for cultivating cells and producing biologics, are also a great investment, having been used by 64.2% more facilities since 2006. Unlike traditional stainless steel bioreactors, which require extensive cleaning and validation between batches, single-use bioreactors use disposable bags that are replaced after each production cycle. This reduces downtime and significantly lowers the cost of production, making them an attractive option for both small and large scale manufacturing.
Facilities are also seen to spend almost 20% of their capital on SUS mixing systems, designed for the preparation and homogenization of complex media and solutions. The systems offer significant advantages, especially in switching between different production runs. As more molecules progressed from clinical production to commercial manufacturing, facilities adopted more SUS technologies to support growth of their new molecules.
One of the primary reasons for the high adoption of SUS is the reduction in capital investment required for facilities and equipment. This benefit is particularly attractive to manufacturers focused on enhancing productivity and achieving short-term cost savings. By minimizing the need for expensive infrastructure, SUS allows companies to allocate resources more effectively, contributing to a decrease in overall facility costs. Thus, we see an investment by facilities on low-cost SUS devices.
However, as the industry surpasses the COVID surge, the explosive growth of these established single-use products and devices is beginning to plateau. In 2024, the annual growth rate of single-use bioreactors slowed to between 1.6% and 3.5% CAGR. This percentage is not indicative of declining SUS need, but rather a sign that the market has reached a level of saturation as the industry now looks towards the next wave of innovation to drive future growth.
A new area of focus is single-use control systems. The systems are components within bioprocessing equipment designed to monitor, regulate and automate various aspects of single-use technologies. These are specifically developed to work with single-use bioreactors, mixers, filtration systems and other disposable technologies.
Features of single-use control systems
Single-use control systems include sensors and software that monitor critical process parameters such as temperature, pH, dissolved oxygen, pressure and flow rates. These systems ensure that the bioprocess environment remains within the desired range for optimal cell growth or product formation. They also are easily adjustable to different production volumes during clinical trials or in response to changing market demands.
The systems often include automation capabilities that allow for precise control of the bioprocess. This might involve adjusting the flow of gases, liquids or nutrients automatically based on real-time data, which reduces the need for manual intervention and increases process consistency. They also provide robust data management features, ensuring that all critical data is recorded and stored in compliance with regulatory requirements.
They also have some limitations. First, single-use control systems are designed for one-time use, which can lead to higher operational costs due to the need for new components for each batch. Additionally, although designed to work with disposable systems, integrating single-use control systems with existing infrastructure can be complex, particularly in ‘hybrid’ facilities that operate both traditional and single-use technologies.
Some challenges shared between single-use control systems and other SUS include concerns about leachables and extractables as well as about waste disposal and the generation of plastic waste. The industry is already making early strides to overcome these issues by focusing on durable new materials that are designed to be more compatible with biopharma processes, significantly reducing the risks associated with leachables and extractables. In addition, efforts to create eco-friendly materials and processes have helped mitigate the environmental impact.
The ability of all SUS to adapt to the industry’s concerns while enhancing production efficiency and flexibility has driven their adoption across stages of manufacturing.
Top adoptions for expansion
As production demands continue to rise, nearly half of industry experts anticipate that their current facilities will struggle to keep pace over the next five years. This concern reflects the inherent difficulty in facility planning, where predicting future capacity needs often reveals the inevitability of outgrowing existing infrastructure. Historically this has been a persistent trend, but SUS are emerging as a pivotal solution to overcome this.
SUS have been frequently cited by industry leaders as a key strategy to avoid capacity bottlenecks. The inherent flexibility of SUS allows for rapid scalability, enabling facilities to adapt more swiftly to changing production demands without the need for extensive infrastructure overhauls. By minimizing the reliance on fixed stainless steel systems, which require significant space and extensive validation processes, SUS offer a more adaptable and cost-effective approach to facility expansion. This flexibility not only mitigates the risk of outgrowing current facilities but also provides a pathway for more efficient and agile manufacturing operations in the future. As such, SUS are increasingly recognized as an essential component in the strategic planning of facilities aiming to stay ahead of capacity constraints.
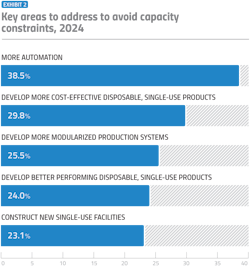
Automation is also seen as a critical factor in alleviating capacity constraints (Exhibit 2), as it allows for more efficient and consistent bioprocessing operations. We expect to see the integration of automation in
SUS via SUS advanced digital monitoring and control technologies. These will allow real-time oversight of critical process parameters and enhancement of both process control and product quality. Furthermore, automation will reduce the likelihood of human error, leading to more reliable and reproducible results. As facilities grow in size and complexity, the integration of advanced automated SUS becomes essential for maintaining operational efficiency and scaling up production.
Following the advancements in SUS, the industry is showing interest in modular bioprocessing units and single-use facilities as a solution to capacity constraints. Modular systems consist of connectable, portable cleanrooms or isolator units that can be quickly assembled and become operational within weeks or months. Offering similar benefits to single-use facilities, these systems provide a flexible, scalable and rapid solution for expanding production capacity.
The concept of ‘plug-and-play’ factories, where entire production lines can be easily replicated and deployed, is gaining traction. Companies like Cytiva, G-Con and Pharmadule are at the forefront of developing modular bioprocessing units that function as fully disposable, single-use systems within portable, self-contained trailers or cleanrooms.
These modular systems are particularly advantageous for applications that require urgent and rapid responses, such as epidemic vaccine production or biodefense. They are also ideal for scenarios where short-term demand is expected, or where high safety standards necessitate the quick setup of on-site facilities. The adoption of modular platforms in commercial manufacturing is expanding. Our recent survey found that over 5% of bioprocessing facilities reported plans to evaluate upstream modular bioprocessing units within the next 12 months.
Future outlook of SUS
The adoption of SUS in the biopharma industry has dramatically increased, especially in response to the demands of the COVID pandemic. These systems have become essential across various stages of production. SUS have been integrated into a wide range of bioprocesses, from upstream operations like mixing and bioreactor use to downstream processes such as purification and fill-finish.
However, the adoption of SUS is leveling off after the first market penetration. The high cost of disposables is a major concern, especially as manufacturers increasingly weigh the long-term financial implications of using SUS. Although the industry has made strides in addressing earlier concerns such as breakage and the risks associated with leachables and extractables, these issues still linger in the minds of many bioprocessing professionals.
Furthermore, the durability of SUS remains a critical issue, with the potential for bag breakage and the subsequent loss of valuable production material being a significant deterrent. Companies that have invested heavily in traditional equipment may also be reluctant to switch to SUS, particularly when considering the limited scalability of these systems across a broad range of production volumes. The conservative nature of the highly regulated industry further complicates the situation, as any changes to established processes or product lines often require extensive validation studies, regulatory filings and costly testing. This hesitancy to adopt new technologies can slow the pace of SUS integration.
Looking to the future, the continued growth and standardization of SUS will depend on overcoming these challenges. Innovations in material science, recycling and scalability will be essential. Moreover, as automation becomes increasingly integrated into biomanufacturing, SUS will likely evolve to include more automated processes. This shift will not only reduce operational costs but also improve process consistency and product quality.
SUS are also expected to play a critical role in the development and production of advanced therapies, such as cell and gene therapies, that require highly specialized and flexible manufacturing processes.
If SUS systems can continue to evolve and meet the industry’s needs of cost savings, improved profitability and support of complex therapies, they are likely to become fully standardized, ultimately enhancing access to cutting-edge biotherapies and contributing to the broader advancement of biopharma manufacturing.
About the Author
Ioanna Deni
Research Scientist, BioPlan
Ioanna is an experienced a Quantitative Market Research Analyst, completing her Ph.D. in Biomedical Sciences and demonstrating extensive experience in biopharmaceutical life science research. Her research background includes quantitative and qualitative market research expertise as an AI and healthcare specialist.