Almost twenty years ago, the pharmaceutical industry began its ruminations on the potential of shifting from a batch-based to a continuous manufacturing platform for pharmaceutical drugs. Despite being used broadly in the food and chemical industry, the pharmaceutical industry had not yet taken the leap.
Since that time, the supply chain and marketplace have truly gone global and survived the major market disruptions of a worldwide pandemic. Looking back at the drivers for pharmaceutical continuous manufacturing (PCM) what, if anything, has changed, and what does the future hold for PCM?
Looking back: A different regulatory landscape
In 2002, the predominant perception was that pharma manufacturing and quality sensibilities were better suited to batch manufacturing. Manufacturing work centers were designed to support individual lot or batch separation and batch manufacturing naturally supported this approach, limited by the individual capacities of the equipment used for manufacturing. Because the volumes of most pharmaceutical products were much smaller than chemical processes, batch processes were better sized and suited for pharma operations. Most processes were first developed in a lab where operations are batch-oriented because quantities are typically small for early process development.
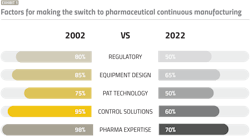
Based on our discussions with drug sponsors and CMOs over the years, Exhibit 1 highlights the core issues voiced by industry, and their evolution. While the factors for making the switch to PCM are the same today, we’ve made progress in reducing the overall risk.
In 2004, the FDA took a big step to move toward a new framework built on scientific understanding as the foundation for drug development and quality. At the same time, the FDA issued its guidance on process analytical technology (PAT), advocating a framework for drug sponsors that would gain better throughput for their process via in-line control. The FDA sweetened the pot by issuing its guidance for drug sponsors looking to leverage real-time release testing (RTRT), pushing for a move to continuous manufacturing and in-line monitoring.
These measures were complemented by landmark harmonized guidances Q8, Q9, Q1O and Q11 from the International Council for Harmonization (ICH) that attempted to provide direction for the adoption of quality by design (QbD) and risk-based quality for small and large molecules.
Together, these guidance documents offered a road map for the industry to move to more insightful drug and process development based more upon process under- standing and less on inspection and testing.
Views on risk evolve
Twenty years ago, the biggest hurdle consistently voiced by the industry in pursuing pharmaceutical continuous manufacturing was CMC regulatory uncertainty. As a risk-averse industry, the appetite for blazing a new regulatory pathway with the FDA was limited enough to keep organizations from looking seriously at PCM. In 2019, FDA issued its guidance on the quality considerations for continuous manufacturing which, for the first time, provided insight into what the industry would have to do to demonstrate an effective control strategy for a continuous process.
In July 2021, ICH issued its draft guidance, ICH Q13, on continuous manufacturing to define to an even greater extent the criteria — and in some cases, approaches — to be used for characterizing continuous manufacturing, thus arming the industry with a much clearer picture of what the EMA and FDA expect in terms of drug process development. Despite this important guideline, there is still no clear harmonization across all markets for what health authorities would expect to support a pharmaceutical continuous manufacturing process submission.
The virtues of PCM
Pharmaceutical continuous manufacturing provides several significant advantages over conventional batch manufacturing. Unlike conventional batch processes, the development and scaling up of the process is greatly streamlined. It isn’t necessary to transfer a process to larger capacity equipment as the program moves to late-stage pivotal clinical studies and commercial launch because greater volumes can be achieved simply by running longer.
While the complexity of characterization for PCM unit operations may be higher than for batch processing, there is no need to scale the process up. This translates to shorter development times and lower regulatory CMC risk as the materials being manufactured across all clinical phases would fundamentally come from the same process. While the initial control strategy may rely on a more sophisticated analytical control strategy — including off-line, at-line and in-line testing — once that is done, fundamentally the drug sponsor has greatly simplified any health authority’s review risk.
Couple this with the findings from a recent survey by FDA that pointed to PCM-based programs realizing faster times to market and regulatory approval vs. conventional batch submissions, and the business case for PCM becomes even more compelling.
Looking beyond the shop floor, PCM gives drug sponsors the ability to respond more quickly to demand variations in the marketplace and not incur the additional sunk cost of carrying inventory. The average pharma company holds inventory for 180 days or more, representing up to 50% of their working capital. Freeing up these resources allows drug sponsors to invest in strategic initiatives that drive their overall business performance and competitiveness.
Implementing PCM brings the industry one step closer to ‘on-demand’ manufacturing and provides tremendous flexibility to adapt to market opportunities, along with unprecedented supply chain flexibility.
What still needs to be done
Several hurdles still need to be overcome for pharmaceutical continuous manufacturing to gain a larger foothold in the industry. Equipment design is evolving, thanks to feedback from industry. Continuous manufacturing differs from batch manufacturing in that the material transfer rates between unit operations must be the same to ensure controlled processing within each piece of equipment. For some low-dose processes, the industry bumped up against the capability of feeders to maintain the accuracy and precision requirements to support the synchronized material transfer requirements. Equipment manufacturers are innovating to address universal operational considerations such as equipment cleaning and changeover, and to meet all the operational requirements of PCM.
Equipment suppliers are now able to provide fully integrated finished drug product continuous manufacturing lines. More importantly, these lines are designed to integrate third party equipment. Equipment vendors are trying to make the capital investment more attractive by providing off-the-shelf integrated unit operations that are available in modules, eliminating integration challenges between equipment.
Today, a drug sponsor can purchase a modular platform that includes continuous processing beginning with powder feeding of APIs and excipients through coating. Some suppliers have platform formulations providing continuous manufacturing equipment specific to direct compression formulations. These efforts prove that the industry is listening and working to simplify the equipment integration component of a PCM approach.
The need for integrated PAT and control solutions The pharmaceutical industry is in the early stages of implementing its digital road map. One of the bigger challenges associated with pharmaceutical continuous manufacturing remains the ability to integrate and capture data from PAT and other intelligent equipment parameters to analyze and manage the equipment, sensors and data in an effective GMP-compliant way which also allows the industry to efficiently pursue strategies such as real-time release testing.
Twenty years ago, there were no fully integrated PAT solutions for industry to adopt, making the transition to PCM a true development exercise. Today there are several new control systems available to address the control, data acquisition and multivariate analysis required to establish the process design space and accommodate PAT for key unit operations. Defending the final control strategy is a core component of any drug’s CMC argument and is also required for drug manufacturers to be able to realize process transparency resulting in higher yields and higher process predictability.
These systems also can do the required instrumentation management that allows the industry to take advantage of innovative new applications of near- infrared and Raman spectroscopy.
These analytical tools have the potential to measure the product’s final critical quality attributes (CQAs) — such as potency — in line and eliminate the need to sample and test on release. Integrating these technologies in line during the manufacturing process requires managing and applying the appropriate configuration and calibration standards to defend the method being in a state of control and the data as GMP. Fully integrated solutions greatly simplify the complexity of deploying PAT in PCM, but we need more of them to help drive PCM adoption.
Looking ahead
In 20 years, the pharmaceutical industry has made strides to realize the promise of PAT and PCM. The advantages, while understood back in 2002, did not yet benefit from mature equipment, control and regulatory solutions available to industry and to health authorities. Adoption has been slower than predicted but the industry and its supplier ecosystem have made the advancements necessary to make PCM a viable reality.
We see that for pharma to adopt PCM, it must move from being a development solution to a mature commercial solution. Modular solutions being provided by suppliers help significantly in addressing the integration challenges of various unit operations and industry is gaining a greater level of comfort with the expertise required to move along its digital road map.