BioProcess Control: What the Next 15 Years Will Bring
Mammalian cell culture bioreactor processes are difficult to characterize and they demonstrate variability between batches. Without sufficient online detection and control, variations can lead to a high number of failed batches.
To eliminate batch variations, many biopharmaceutical manufacturers are moving beyond today’s “quality-by-inspection” methodology and adopting Quality by Design (QbD) methods. These methods center on measuring more variables online and increasing the sophistication of automation.
In-line process variables that could not be monitored in the past, can now be measured, analyzed, and used for advanced control schemes. Producers can use this additional process data to better understand how to reproduce consistent batch quality and yield, and to advance toward the goal of consistent batch-to-batch profiles.
Measuring cell health and metabolites can reduce risk, increase operators’ confidence level and provide more consistent results. Online detection of abnormal consumption and apoptosis would allow operators to terminate bad batches at an early stage. For instance, feedback control of glucose concentration enables a fixed, low concentration of glucose in a fed batch bioreactor that cannot be replicated with periodic, manual or open loop feeding.
Fixing the glucose concentration at a low value holds the potential to increase the quality of a glycosolated product by reducing uncontrolled glycation [1] and improve efficiencies of nutrient utilization [2]. The pre-requisite to monitoring is improved sensors. Analyzers coming onto the market can take key measurements online continuously or at-line periodically through automated samplers to optimize product quality and yield.
Measurements that, until now, were intermittent and off-line, can now be made periodically or continuously, online. In some cases, users can choose among several different techniques to analyze a component. Some online and at-line sensors that can fulfill bioreactor processing needs are already on the market and need only to be adapted to bioreactors. Other sensors specifically for use in microbial fermentors and cell culture bioreactors are in late stages of development. This article will review the next generation of sensors that will enable closer control of biopharmaceutical cell culturing. Most sensors discussed here are expected to be in common use within the next few years. Part 2 of this article, to be published next month, focuses on bioprocess modeling.
Dielectric Spectroscopy
The dielectric or passive electrical properties of cells can be exploited to measure viable cell density online. In an induced electrical field, an intact cell membrane is a physical barrier to ion migration. The induced electrical field turns each cell into a small capacitor.
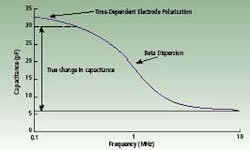
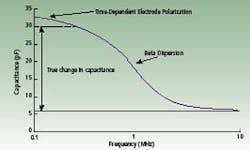
If the direction of the electrical field is alternated over a range of frequencies, the capacitance of the cell varies from a low frequency capacitance plateau to a background capacitance due to water at high frequencies. Capacitance measured in picoFarads plotted against the frequency of change of the electrical field, measured in MHz, is called a beta-dispersion spectrum. This curve is affected by electrode geometry but can be normalized.
The end result of the normalization is a similar curve based on a dimensionless relative permittivity verses frequency. However, the electrode is subject to a time-dependent electrode polarization. The polarization effect is an increase in permittivity at low frequencies. The effective measurement frequency range is limited at low frequencies by the polarization effect and at high frequencies by background capacitance.
Older analyzers measured a single low frequency as close as practical to the electrode polarization effect. Newer analyzers take random measurements at frequencies between 0.1 and 15 MHz and use a non-linear least squared fit to generate the entire frequency spectrum. Cell suspension bio-volume, cell radius, membrane capacitance, internal conductivity of cells, homogeneity of cells, and medium conductivity parameters are computed from the spectra [3].
Although a computation-induced measurement lag exists, it is not significant compared to cell culture growth rate. Cell culture viable cell counts and bio-volume measurements from dielectric spectroscopy sensors have been found to match off-line measurements.
Encoded Photometric Infrared Spectroscopy
In the past, infrared analysis has not applied in the pharmaceutical industry as frequently as in other industries. New technology like Encoded Photometric Infrared analysis and FDA’s PAT and QbD incentives increase the importance of this technology to the pharmaceutical industry.
Any infrared senor used online in a cell culture bioreactor requires the analysis to be rapid and sensitive. The instrument must be stable and not require frequent re-standardization or calibration updates. Dispersive IR analyzers split the light source into individual frequencies before the light passes through the sample. Wavelengths over the entire spectrum are measured sequentially. The analysis is slow and only portions of the spectrum are needed for a given analysis.
Non-dispersive IR analyzers send the entire light source through the sample and use fixed frequency pass-band filters to select desired frequencies. This technique is most often used in field instruments for a specific analytical function. Although non-dispersive IR analyzers may meet online requirements of speed and stability, the analytical function is limited by the reduction in signal-to-noise ratio as the number of filters is increased [4].
Recently developed encoded photometric infrared analyzers can detect the constituents of multiple frequencies simultaneously. Unfortunately, array detectors that would allow rapid detection of the complete spectrum and are so common in visible light applications are too complex for mass production [5]. Instead, EP IR analyzers modulate the amplitudes of the components of each wavelength of interest in a process termed encoding.
The encoded beam is collected and received at a single point detector [4]. The EP IR analyzer is a non-dispersive measurement where the radiation beam is dispersed according to wavelength after it has passed through a sample. The energy is converted into spectral components and distributed according to frequency over a spinning disk; the encoder. The encoder disk contains dispersed radiation filters to modulate the amplitude of a single wavelength or channel as digital replicas of smooth functions and that render the encoded wavelength components orthogonal to one another. The orthogonal channels differentiate the contribution to the encoded beam caused by the wavelength components in the various channels [4]. The dispersed radiation filters, or modulators, on the encoded disk are specific to each analytical function.
Unlike nondispersive IR analyzers with fixed filters, the modulators can be replaced simply by replacing the encoder disk. Since the encoder disk is the only moving part, EP IR analyzers are suitable for online analysis. Unlike non-dispersive IR analyzers the analytical function is not limited by decreasing signal-to-noise ratios. Unlike dispersive IR analyzers, the sampling capability is as fast as 100 Hz. Online analysis of nutrients and metabolites in cell culture bioreactors is currently in development. The analysis must be split among several EP IR sensors; each with a unique encoded disk.
Hypothetical beta dispersion spectrum of a cell suspension showing the
contribution of electrode polarization at low frequencies.
A single analysis function can measure glucose, glutamate, glutamine, proline, lactic acid, ammonia, and dissolved carbon dioxide in the cell culture medium. Only dissolved carbon dioxide can be measured online using currently available technology. Dissolved carbon dioxide sensors have lag times that make them impracticable. Amino acid IR fingerprints overlap, so analysis of all 20 amino acids in the medium must be grouped in as many as five EP IR analyzers.
Analyzers outputs must be regrouped in a way that functionally makes sense in a cell culture. More development work is needed here than in the glucose analysis, but successful application opens the door to amino acid feeding strategies. Several authors employ MPC control of concentrations of several amino acids to prevent starvation induced apoptosis in Chinese Hamster Ovary cell cultures. Direct measurement by EP IR analyzers would eliminate the need for state estimation of the amino acid concentrations proposed by these authors.
An early and sensitive indicator of the onset of apoptosis is the change in concentration of auto fluorescent NADH as mitochondria are disrupted [6]. It may be feasible to use EP IR to detect the fluorescence in a bioreactor provided optical density can compensate for the volumetric increase in HADH concentration associated with the increase in cell density. Infrared spectra are not direct measurements. Rather, spectra must be correlated to known data in order to predict unknown data by way of a chemometric analysis. The training and validation data sets for the correlation must represent the full range of variability that can occur in a bioreactor.
Automated Multifunction Analyzers
Multifunction analyzers are an automated or robotic combination of enzymatic, amperometric, potentiometric and coulter counter or CCD camera analyzers. The list of possible measurements includes; sugar and amino acid substrates, metabolic byproducts, dissolved oxygen and carbon dioxide, pH, cell density and viability, sodium, potassium, calcium, phosphate and osmolality. Installing online sensors for all these measurements is not practical and in some cases is not feasible. Osmolality can only be inferred by a cluster of online sensors while at-line multifunction analyzers measure osmolality with the “gold standard” freezing point depression test.
At-line analysis does have a down side. Samples must be taken from the bioreactor manually. Help may soon come from autoclavable multi-point auto-samplers. If the sample volume is small, samples from multiple bioreactors can be taken at shorter time intervals. A manual sampling may be every 12 hours while an auto-sampler might run on a 4- hour schedule. Closed loop, negative feedback control with an auto-sampler is feasible if the sample interval is 25% of the dominant system response time.
Online Product Analysis
Most product analysis for quality and quantity is performed on manual samples, with assays that are specific to that product. In single product, dedicated facilities, automating those assays is possible using specifically developed systems. This level of sampling and analysis may come at a high, but warranted, cost. In multi-product facilities, the variability of assay techniques may hamper the ability to automate product analysis.
In cases where similar assays may be used across multiple products, such as using Protein A binding assays for quantitating monoclonal antibodies, automating assays is achievable. Autosamplers can be interfaced with HPLC systems to allow direct sampling from the tank but samples require special treatment to remove cells and debris that will clog the column. On the horizon are analyzers which have the ability to quantitate monoclonal antibodies in a “dirty” sample as well as metabolites and cell count. The ability to monitor the quantity and quality of a protein, or even a class of proteins, online is technically feasible using near-infrared technology, but requires development time and resources.
Other technologies that take advantage of molecular binding to a specific substrate to produce a measurable signal, such as surface plasmon resonance (SPR), may hold promise in developing online sensors allowing the user to determine the quantity and quality of a product before it ever leaves the tank.
References
- Yuk I, Huynh H, Leach K, Shen A, Zhang B, Dutina G, McKay P, Lim A, Snedecor B, Cell culture efforts to reduce glycation in recombinant humanized antibody. The 4th Recombinant Protein Production Meeting: a comparative view on host physiology Barcelona, Spain. 21–23 September 2006 Published: 10 October 2006 Microbial Cell Factories 2006, 5(Suppl 1):P57 doi:10.1186/1475-2859-5-S1-P57
- Marange L and Goochee C F, Metabolism of PER.C6 cells cultivated under fed-batch conditions at low glucose and glutamine levels. Biotechnol Bioeng 2006 May 5;94(1):130-50.
- Yardley J E, Douglas K B, Barrett J, Davey C L, On-Line, Real-Time Measurements of Cellular Biomass using Dielectric Spectroscopy. Biotechnology and Genetic Engineering Reviews – Vol 17, August 2000.
- U.S. Patent No. 6,982,788.
- US Patent No. 5,485,268.
- Toms S A, Muhammad O, Jackson H, Lin W C, Decline in NAD(P)H Autofluorescence Preceeds Apoptotic Cell Death from Chemotherapy. Proc. Of SPIE, Vol. 6009, 60090Q, (2005).