Portable Raman for Raw Material QC: Whats the ROI?
The need to ensure the safety of pharmaceutical raw materials has driven many pharmaceutical manufacturers to outsource raw material testing or to use traditional sampling and laboratory techniques, increasing costs and taxing laboratory capacity. Today, a number of portable analytical technologies are available to allow raw materials to be analyzed right in the warehouse. Among them is Raman spectroscopy [1,2,3], which eliminates the need for sample preparation and allows measurements to be taken, even within glass or plastic, right in the manufacturing plant or in the field.
Each analytical method has its strengths and liabilities. NIR, for instance, can characterize particle size and moisture content, where Raman cannot. However, it requires that users develop calibration procedures and chemometric models specific to each process, material and instrument. Raman, meanwhile, requires the use of algorithms to compensate for its fluorescence signal, which can reduce users’ ability to acquire an interference-free signature for a given material.
This article quickly runs through pharmaceutical manufacturers’ options for improving raw material testing operations for quality assurance and manufacturing efficiency, comparing the relative costs for various scenarios.
Increasing Existing Analytical Laboratory Capabilities
Initially, this may look like the logical way to cope with the extra work. However, when the overall analytical capabilities of the company are taken into consideration and Return on Investment (ROI) calculations are done, it can only be justified if the numbers of samples to be analyzed are very small (perhaps in the order of 20-30 of extra analyses a month). So this option is typically only valid for companies that work with less than 10 different materials and when most of the samples are already being analyzed, so the incremental increase will have a limited impact on its operational costs.
Introducing New Analytical Capabilities in the Warehouse
An extensive study should be carried out to determine the best analytical technique to use with the given material. For example, if the analysis is to be done mostly on crystalline salts such as NaCl, and KBr, vibrational techniques are probably not the best choice. If the requirement is to analyze active pharmaceutical ingredients (API) or standard excipients used in the pharmaceutical industry, either Raman or NIR should be considered as strong candidates for the analysis.
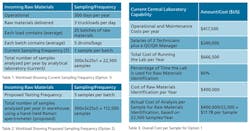
The next few tables compare the specifics and relative costs of Options 1 and 2, using real-world data from three mid-sized pharmaceutical manufacturers based in the United States and Europe. The companies had roughly 100 raw material chemicals that required testing. It shows results for the NanoRam device [4,5,6]. Current operations involve the analysis of a representative number of samples (some bags or drums per pallet), but regulations and corporate policies now call for the incremental analysis of all samples [7].
In Option 1, the cost was in the order of $13-15/sample. It was therefore important to calculate the actual cost and compare it with the cost per sample if all the batches of the incoming raw material were sampled and identified in the warehouse using the hand-held Raman approach. This cost can be assessed based on an estimate of the cost of operating and maintaining a full Central Laboratory capability, the percentage of time the labs were used for raw material identification, and verification and the salaries of the lab personnel carrying out the analysis. Table 3 gives a detailed breakdown.
Phasing Out Central Lab Capabilities Over A Three-Year Period
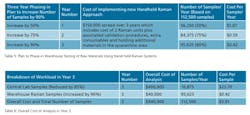
In this third option, central lab capabilities are phased out over three years and replaced by materials identification in the warehouse using handheld Raman devices. In this case, the sampling frequency has been increased to sample all batches, meaning the total numbers of samples increases from 22,500 to 112,500 per year. Table 4 shows the cost of phasing out the testing using Central lab capabilities, by reducing it by 50% the first year, 75% the second year and 85% the third year. It’s also important to emphasize that approximately 15% of all samples will still need to be sent to the lab, because 10% are not suitable for Raman testing and 5% will be needed for confirmation or retesting purposes.
The initial cost of testing in the warehouse is associated with the cost of the Raman units themselves. These particular companies purchased two units, one for warehouse use and a second for the central laboratory, used for method development, validation protocols, implementation of new IQs/OQs, and as a backup unit.
There is also an associated cost for the validation of the different chemicals that need to be analyzed, estimated on the basis of personnel time, cost of reference materials, standard operation procedure documentation (SOP) and technique validation. Another of the parameters included in the calculations was the cost associated with manpower. There was no need for extra technicians in the warehouse, just a reorganization of the technical manpower capabilities. For example, one of the technicians in the central laboratory was also in charge of the analysis in the warehouse. The calculations for all these parameters mentioned is based on a three-year introduction plan, and assuming that 90% of the materials are valid for Raman identification, the plan targets to implement and validate 50% of the materials in Year 1, 25% of the materials in Year 2, and the remaining 15% in Year 3. The remaining 10% of the materials will still be analyzed in the laboratory, as well as one sample per batch for quality control assurance.
The plan assumes the number of samples will remain constant over the 3 years. However, the reduction of samples analyzed by the central laboratory will have a direct impact on the cost per analysis, as the number of samples required to be sent from the warehouse to the central laboratory was estimated to decrease by 85%. It is also worth pointing out that as the number of samples analyzed in the lab changes by 50% in the first year, a reallocation of manpower and some instrument consumables is required, which, in this scenario, is covered by the validation costs.
The phasing in of the warehouse testing over three years, using the two Raman units, is outlined in Table 5, while the total cost per sample in Year 3 is shown in Table 6. The average savings associated with changing to warehouse testing were in the region of $150,000, based on the difference between the reduction of laboratory operational costs, consumables and other factors (~ $250,000), less the initial investment of the two hand-held Raman spectrometers and associated validation procedures, which was estimated to be about $100,000.
For these companies, the extra costs represented an average of approximately $300,000 per year to the operational budget. When the existing central laboratory operational/maintenance costs per year of $417,500 are factored into the calculation, this translates to a total cost per sample of $6.34, based on the increased number of samples from 22,500 to 112,500. However, this amount is significantly lower than the initial cost of $17.78, when only 20% of the samples were tested. A cost comparison of these two lab-based testing scenarios with the handheld Raman warehouse solution is summarized in Table 7.
Although each facility faces unique challenges, this comparison suggests that handheld Raman testing in the warehouse offers pharmaceutical manufacturers a powerful tool to help increase efficiencies and reduce the cost of raw material QC.
References
1. An implementation Perspective on Handheld Raman Spectrometers for the Verification of Material Identity: B. Diehl, C.S. Chen, B. Grout, J. Hernandez, S. O’Neill, C. McSweeney, J. M. Alvarado and M. Smith, Pfizer Inc; European Pharmaceutical Review, Non-destructive Materials Identification Supplement, Volume 17, Issue 5, 2012, http://www.europeanpharmaceuticalreview.com/wp-content/uploads/Raman-Supplement-2012.pdf
2. Portable Raman Spectroscopy for Pharmaceutical Counterfeit Detection: R. Kalyanaraman, M. Ribick and G. Dobler, Bristol-Myers Squibb; European Pharmaceutical Review, Non-destructive Materials Identification Supplement, Volume 17, Issue 5, 2012, http://www.europeanpharmaceuticalreview.com/wp-content/uploads/Raman-Supplement-2012.pdf
3. Fake Pharmaceuticals: Bad Medicine, The Economist, October 13, 2012, http://www.economist.com/node/21564546
4. The NanoRam hand-held Raman Spectrometer: B&W Tek Application Note, http://bwtek.com/products/nanoram/
5. NanoRam: Full Regulatory Compliance for the Inspection of Raw Materials & Chemicals in the Pharmaceutical Industry: B&W Tek Application Note, http://bwtek.com/learning-lab/application-notes/
6. The NanoRam Hand-held Raman Spectrometer: Ideally-suited for the Inspection of Raw Materials and Chemicals Used in the Pharmaceutical industry: B&W Tek Application Note, http://bwtek.com/learning-lab/application-notes/
7. Statistical Solutions: Square Root of (N) + 1 Sampling Plan: L. D. Torbeck, Modern Medicine, Oct 2, 2009; http://www.modernmedicine.com/modernmedicine/article/articleDetail.jsp?id=631722