Pharma’s capital equipment spending has slowed but remains robust, focused on optimizing manufacturing supply chains through highly targeted technology and services purchasing
Pharmaceutical companies operating on a global scale continue to refine their business models, defining and executing strategies that their boards and executive management hope ultimately will lead to market success and sustained revenues. No one can argue that over the last decade or so the pharmaceutical industry has experienced dramatic change, change that has reordered the status quo and added new and challenging topography to the competitive landscape.
According to Price Waterhouse Cooper’s (PWC’s) 17th annual Global CEO Survey, Key Findings for the Pharmaceutical & Life Sciences Industry: “Pharma’s future has never looked more promising — or more ominous.” Certainly to most pharmaceutical professionals that conclusion is an obvious truth, but it does reflect the seriousness of what’s at stake. So while Pharma CEOs are, say the survey’s findings, “confident about their own future revenue prospects,” they are also concerned with economic conditions and the impact of government on growth. Based on interviews with 90 Pharma and Life Sciences CEOs from 37 countries, PWC’s study revealed that executives are working hard to meet the demands of patients and society, acknowledging that growth markets are critical to future success, cost cutting is an imperative and R&D and innovation remain top priorities.
Pharma companies’ chief executives are bullish, say PWC analysts, on their own prospects as far as achieving growth, but not so confident in the industry as a whole in achieving aggregate growth in the face of shifting global macroeconomic forces. Of those Pharma CEOs surveyed, nearly 50 percent expect middle-term growth for their own company while 29 percent feel the same about overall industry growth. This attitude helps affirm the perception that Pharma’s leaders are seeking market and financial success via careful capital investment and attention to operational excellence, understanding that competitive agility and profit will come from investing time, money and effort into enhancing and refining their own operational and organizational resources regardless of this new era of “stable instability” as PWC characterizes the pharmaceutical and life sciences industry. “Risks that once seemed improbable, even remote, have become the norm,” say PWC analysts. “For CEOs across the world, ‘expect the unexpected’ has become the mantra.” So where is Pharma finding its agility? Increasingly they are recognizing it can be mined from within operations and from from the operational excellence of carefully selected partners.
Traditionally, “innovation” in the Pharma universe was understood as a given company’s ability to develop new therapies and compounds and to keep its R&D pipeline filled with potential blockbusters. But most Pharma CEOs are well aware that no matter how much they spend on R&D, creating opportunities to innovate a profit-sustaining blockbuster are getting more rare every day.
But this doesn’t necessarily translate into fewer drugs being developed. In the study “Trends in the 2013 Pharmaceutical Pipeline,” from the American Health & Drug Benefits (AHDB) association, the study’s authors noted that judging by the number of drugs that are currently in the pipeline, the nature of drug development may be changing, but is not showing real signs of slowing down, despite the many marketplace uncertainties and recent economic instability in the U.S. and around the world.
According to Anthony D. Sabatelli, a pharmaceutical intellectual property expert with Dilworth IP, “the FDA is optimistic about the trends from the 2013 numbers. The Agency reports that the ’27 NMEs approved in calendar year 2013 is similar to the average totals approved in the past decade.’ The Agency also reports that from 2004 through 2012, CDER averaged 26 approvals per year and that the 39 approvals seen in 2012 ‘was an unusually high number,’ however, others are less complimentary and have cited higher development costs, lower rates of return on these costs, and lower peak sales, as possible causes for the less than stellar performance of 2013.”
In Sabatelli’s January 2014 blog “The Scorecard – Fewer New Drug Approvals in 2013: What’s in store for 2014?” he notes that one cannot readily read a trend into 2014. “If anything, the more pertinent information from the FDA is that the number of new drug applications has not been consistently and significantly increasing.” Sabatelli said that from 2004 through 2012 the FDA reported an average of 34 NME applications have been filed per year.
And while the pursuit of blockbusters may or may not be waning, ABDH and others recognize that the more apparent growing trend in drug development “is the accelerating rate of growth of specialty drugs in the pipeline, with an increasing role for the use of genetics and biologic processes.”
By 2018, consumer and payer spending on specialty drugs is expected to exceed the spending on small-molecule drugs.” From another one of its studies PWC found there are about 460 therapies for rare diseases in the pipeline.
MARGIN-PRESERVING TECHNOLOGY
Pharma’s executives, say PWC analysts “believe that technology is transforming the sector and they are using ‘strength in innovation’ to make the most of it.” Yes, Pharma is finding strength in innovation and investing in the technologies to fuel it. But recall that the specialty drugs and biopharmaceuticals that are framing drug development require “high cost” and more likely highly optimized processes to help them get them to market and Pharma’s capital spending behaviour is reflecting the ongoing enhancement and redevelopment of pharmaceutical processing capacity to improve throughput and quality while supporting cost containment.
Financial powerhouse GE Capital recently completed its “Healthcare Industry Economic Outlook Survey” after surveying executives at 86 middle market healthcare industry services and product firms, many of them prominent pharmaceutical companies. While GE Capital’s findings include companies outside pharmaceutical manufacturing, the results do provide a benchmark to judge attitudes, aspirations and capital spending trends within the sector. GE Analysts found the most “significant” insights to emerge from the study include the opinion that healthcare firms service and product companies are facing margin pressures due to challenges growing revenues while coping with the costs of business, and that the majority expect capital spending to remain flat with about 30 percent planning a modest (1 to 5 percent) increase. A snapshot survey of Pharmaceutical Manufacturing’s readers found that out of the readers’ responses half indicated capital spending will increase in FY2014 and another 36 percent responded that spending would remain about the same.
GE Capital says capital expenditures are likely to focus on replacement of existing equipment, infrastructure maintenance and new equipment purchases. GE Healthcare analyst and senior vice president Savant Ahmed illuminates the point: “If you look at pharma as a whole, we typically finance mid-cap or medium-sized companies. Taking a look at pharma as a whole, [we] are seeing CapEx spending [will be] steady to maybe coming down over time. And the makeup of that is changing as well. In the sense of what they’re spending money on, it’s changing. And then it varies from what’s going on in large Pharma versus what’s going on in biopharmaceuticals.”
Although recent industry studies reveal a general leveling off with large-cap Pharma moderating capital spending, spending activity from mid-cap firms, like the generic producers, CMOs and the CRDOs may be ramping up to capture the business that’s being shed by larger pharma. “I think what you’re seeing is actually fairly interesting,” says Ahmed, “We are seeing the same trend now spreading to [companies like] Teva and Activis — as well as more of the [biopharmaceutical) pharma side as well.”
Ahmed notes those firms are pretty big in their own right and are carrying some of the same burdens that large-cap Pharma firms used to have exclusive rights to. “With those larger companies, you see CapEx coming down. I think the lag is being picked up by other companies, but it’s being done in two ways: One, they’re just taking over the capacity that large-cap pharma is getting rid of. So it’s not new CapEx. It’s basically transfer of assets, so the implications of that are different than if it was like new business.” Ahmed explains that companies are putting in new capacity in terms of manufacturing, but that new capacity is not being located as much in the U.S., other than the odd biological manufacturing plant.
But as mentioned, and most perceive already, CapEx spending of late is not being driven by the construction of new, large commercial solid dose plants in the U.S. “The growth in terms of sheer volume is all coming from emerging markets,” says Ahmed.
PERFORMANCE SHOPPING
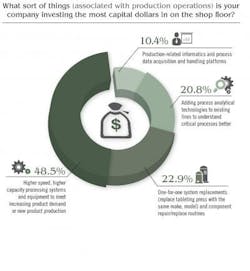
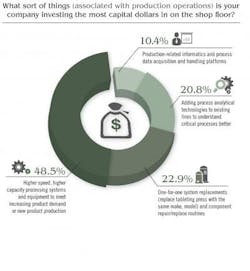
Price Waterhouse Cooper’s study noted that “In other research … the most successful CEOs are doing three things to ‘industrialize’ innovation, i.e., make it repeatable, dependable and scalable.” Here, PWC study authors noted that respondents were busy focusing on innovation in all forms, but especially telling was this statement: “We think it’s important to recognize that innovation means more than new product development. It can also improve processes or create new services or business models.” Indeed, Pharma’s capital spending is driving innovation but innovation tied to increasing production, boosting overall efficiency and improving product quality. GE Capital’s study revealed that 28 percent of firms in the healthcare sector will be considering additional financing for equipment. When it comes to production-oriented CapEx investment, Pharmaceutical Manufacturing’s reader survey found that some 45 percent of respondents are investing the most capital dollars on “higher speed, higher capacity processing systems and equipment to meet increasing product demand or new product production.”
Ahmed noted that a big chunk of CapEx is not necessarily going into manufacturing, but into IT systems — especially those that support research.z
To anyone overseeing a global network of manufacturing operations, managing these assets effectively is incredibly complex, especially when it means gathering data across quality management systems, batch records, process controls, etc., and integrating it all to higher level informatics including MES and ERP. In this modern era most pharmaceutical companies can not really function without adequate, comprehensive information systems that are well integrated to all aspects of operations and manufacture. However, for large-cap Pharma, GE’s Ahmed perceives CapEx spending has run its course. “If you talk about manufacturing specifically, I think that cycle is pretty much done. The large pharma companies have done a fairly decent job. I used to work at Merck until 2005. Even then they were more than midway through putting in a lot of electronic systems; IT systems and quality control systems within their manufacturing facilities,” says Ahmed.
Ahmed did concede that perhaps for large-cap Pharma that may be true, but for smaller companies IT spending has lagged to a certain degree but that spending was going more toward data analytics than just control and similar tactical information and data managing functions.
Are broader information technology integrations driving capital expenditures? Ahmed agrees, but other spending drivers are at work, ones that are happening outside of manufacturing. “There’s a big amount of CapEx spending going on. Take a look at Pfizer. They’re doing $2 billion of CapEx every year. Merck is doing something similar. So there’s a lot of [spending] going on. My sense is that a lot of that CapEx is actually going into more research equipment side of things than the manufacturing side. What I mean by that is [companies like those] are buying new high performance genomic systems and some of them are very expensive. That’s where a lot of this money is going into right now.”
A BETTER BALANCE SHEET
It’s apparent that Pharma CFOs, especially Big Pharma’s, are looking for ways to balance the balance sheet better. As one Pharmaceutical Manufacturing contributor put it, the bias used to be toward a CapEx-intensive internal management and control of operational assets, as opposed to an OpEx-intensive strategy that organizes a supply chain of operational assets and pays for it out of operating budgets.
Ahmed, speaking within the context of the information and data gathered from GE Capital’s life science and pharmaceutical companies explains that mid-cap companies working with mature off-patent drug products are increasingly outsourced, and increasingly virtual in the way they accomplish manufacturing. Biopharmaceutical companies, on the other hand, understand that the process is the product, and so are tending to be more CapEx oriented and vertically managing manufacturing, as a core competency. “If you look at the genetic companies that are in our portfolio, they typically do their own manufacturing because they consider being vertically integrated is a core skill and a cost advantage to some extent.”
In the end it is about flexibility and agility. Ahmed, recalling his days at Merck, says companies like his employer ran into massive problems being CapEx heavy, and not likely to get into that situation again: “So in their view, [they say] ‘we are better off … letting someone else handle a big chunk of manufacturing. It gives us the flexibility as volumes shift, and somebody else can do it much cheaper than we can, especially if they put it in a low-cost manufacturing environment.’”
Better capacity utilization and strategic process optimization prompting continued investment
If there is one thing Pharmaceutical Manufacturing’s (PhM’s) readers have in common, it’s their focus on operations. Our February 2014 Signet audience poll revealed that nearly 90 percent makes final supplier decisions or plays a key role in making recommendations to their organization’s buying team. In July, PhM’s editorial team surveyed its readers in attempt to gauge, or take a snapshot of capital spending plans and attitudes. Unfortunately, response to our survey was limited to 50 readers. Granted, this number of respondents does not engender much of a statistical base to make harder data-based conclusions about the industry at large, but of those intrepid and loyal readers more than half had spending authorization ranging from $26,000 to more than $100,000 — and of those respondents, 28 percent were authorized to spend more than $100,000.INNOVATION, IMPROVEMENT
With the exception of three respondents, all those responding to the question, “How important is process innovation or improvement to your organization or business unit?” acknowledged that innovation and improvement were priorities, regarding it “important,” (32 percent) to “very important” (64 percent).
For the most part, many of our readers are tasked with keeping things going operationally on a day-to-day basis. And although respondents may recognize the strategic, long-term imperative involved in pursuing “process innovation, “day-to-day” priorities and goals may be different than other longer-term operational goals. Half of all respondents indicated that “increasing production” was a top daily operational priority. Thirty-eight responded that “boosting overall efficiency” was a day-to-day goal for manufacturing operations and another 32 agreeing that “improving product quality,” was top goal for daily operations.
It would likely be exceedingly tough to pursue important day-to-day operational goals when overall operations are not generally being kept up. When asked, “Given your current facility/operational profile, how would you generally characterize your production assets and manufacturing systems?” half responded “Dated but well maintained and cost-effective to operate;” followed by 16 agreeing that systems were “Current, up-to-date, meeting global productivity and quality standards.” That left eight noting, unfortunately, that their production equipment and manufacturing systems were at the “End of their lifecycle and increasingly costly to maintain operational excellence.” This suggests that most existing capacity (at least for our modest group of respondents) is being kept in good order, but there are some out there who are dealing with the consequences of capital spending habits that may have been something less than enough, over time, to keep operations current with best, or at least better practices.
RECENT CAPITAL INVESTMENT PERFORMANCE
We asked readers, regardless of their spending authority, how they might rate their company’s capital spending/investment performance when it comes to improving production infrastructure. Fourteen, or 28.6 percent, indicated they felt it was “Ample and a high priority; focused on meeting present and future business goals. Just about half of respondents rated capital investment performance as “Adequate, keeping pace with professed productivity, safety and quality-associated business goals.” That left 12 finding that capital investment by their companies was “Limited, consistently conservative, seemingly a low priority,” an opinion that is not necessarily harbinger of poor operational performance, but one that may indicate a rising risk profile associated with operations.
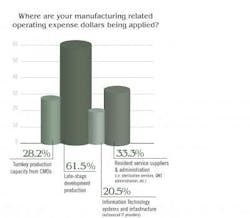
The spread of responses is interesting and perhaps notable because most recognize that late stage development is so critical.
Lastly we wanted to get a better handle on the trend identified above so we offered this final query: “Considering the trend of seeking new opportunities for shifting capital expenses to operating expenses, how would you characterize such activity (if it is occurring) at your company?” Here’s how responses broke out:
• Traditional; accounting practices are consistently applied using standard norms to assign capital and operating expenses (31.3 percent).
• Increasing; we are seeking partners and suppliers that support this operational/financial necessity (27.1 percent).
• Random; not necessarily a part of any deliberate strategy, but being used to help the balance sheet (22.9 percent).
• Piloting; we are beginning to explore the possibilities and trialing suppliers and vendors that support formalized operating expense accounting initiatives (18.8 percent).
The spread of responses point to the fact that at least some of PhM’s operational leaders are engaging in deliberate initiatives, while others not so much and still others either experimenting or encountering the practice in day-to-day operations.