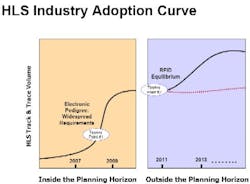
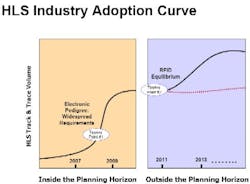
Counterfeiting continues to be a problem of crisis proportions for the healthcare and life sciences (HLS) industry, and increasing regulations and retailer mandates are forcing HLS organizations to make large-scale investments in track-and-trace, electronic pedigree, and RFID-enabled systems.
During a recent Healthcare and Life Sciences Industry RFID Summit sponsored jointly by the Healthcare Distribution Management Association and the National Association of Chain Drugstores, acting FDA Commissioner Dr. Andrew C. von Eschenbach said, Some of you must, all of you should , in reference to the FDAs recent removal of the stay on the Prescription Drug Marketing Act and the resulting requirement for non-authorized distributors to provide and maintain drug pedigrees. Certainly, a continued call to action for the entire industry as it progresses with RFID adoption.
Where We are Today and What We Have Learned
Nearly three years after the FDAs February 2004 report first announced its support for RFID adoption and enhanced anti-counterfeiting measures, supply chain security remains at the top of the agenda for the pharmaceutical industry. Thats not likely to change in the foreseeable future, as the industry has a long way to go to achieve zero gap security. Some leaders and innovators have proven clearly that RFID and other track-and-trace technologies can achieve the visibility necessary to detect and prevent counterfeiting, theft and diversion. In other words, they have moved out of the laboratory and into the piloting phase and, in an increasing number of cases, into real world supply chains.
For instance, we know from experience that electronic pedigree (ePedigree) systems can operate effectively in fully functioning supply chains that reach from manufacturing plants, through distributors facilities, to pharmacy shelves. Large pharmaceutical manufacturers, wholesalers, and major retail pharmacy chains have launched electronic pedigree and RFID serialization pilots over the past year and we fully expect these initiatives to become even more widespread in the next six to 18 months as organizations gear up for the 2009 California electronic pedigree mandate.
Investments in RFID adoption continued to increase in 2006 and we expect even larger investment growth in 2007. Many of these investments will be belatedly for some organizations in electronic pedigree, RFID, and other types of track-and-trace pilots.
Thats the good news.
The bad news is that during 2006 progress has been less than anticipated. Primary issues that industry and solution providers cite include continued debates regarding prioritization and scope definition for item level serialization; specifically what drugs, which locations, which frequency, and what technologies RFID, bar code, 2-D bar code, or some combination thereof? Other issues include trading partner collaboration, U.S. vs. global concerns, evolving standards, and, last but not least, the cost burden.
Return on investment (ROI) always is an important consideration (particularly in a time of tightening margins and shrinking pipelines), but patient safety transcends the point. Industry, government, and consumers have all rallied around this point. Brand integrity, business process improvements, and supply chain efficiencies are also key drivers, but again, patient safety is priority number one. Therefore, we believe that its inevitable that cases and items will be tagged. The regulatory environment certainly points in that direction and the existing infrastructure can be further enabled to make it a reality.
Key Factors Influencing Adoption
Four driving forces have had and will continue to have the biggest impact on track-and-trace and RFID adoption rates in the industry:
Together, these factors present the industry with something of a conundrum. For instance, state-level policy initiatives to establish and maintain pedigree information are compliance-driven and aimed at wholesalers primarily. However, they radiate out to downstream and upstream trading partners through existing relationships and California pedigree law specifically calls for the manufacturer to initiate pedigrees.
Then there are the voluntary policy initiatives such as the FDAs call to action driven by the 2004 report and their continued reinforcement that electronic pedigrees and the use of RFID as a means to provide secure supply chain track-and-trace capabilities are the best methods for protecting the US drug supply. This means that each actor in the pharmaceutical supply chain has a different set of drivers and priorities and a different level of urgency. Thats one reason why many question the role of RFID in near term compliance-driven initiatives.
Policy Decisions
The FDAs 2004 guidelines inspired some initial track-and-trace pilots, but as progress has lagged, several state governments in the United States have pushed harder to directly address the distribution aspect of the HLS supply chain. Florida, California and Nevada have moved forward with pedigree laws. Its generally accepted that more states will join in, which could prompt further FDA activity. The FDA has continued to reinforce its initial guidelines and work with industry to mobilize a multi-layered strategy and critical capabilities including mass serialization, e-Pedigree, and authentication.
Trade Relations
Trade relations have acted as both a contributor to and an inhibitor of industry adoption efforts. We believe trade relations are the key to finding business value in the concepts of zero-gap security and 100 percent patient safety. Pharmas, distributors and retailers have key roles to play here, as do technology providers, consultants and industry groups. The tough issues around data sharing, visibility, and security can be resolved only through collaborative models and practices.
Process Change
Process change is still another variable and, obviously, the most operationally oriented one. Specifically, how will track-and-trace impact existing processes? How do these changes integrate or potentially conflict with existing compliance requirements? Of course, the issue isnt just what will be impacted and what needs to change within the enterprise, but also how will new processes be implemented across the supply chain?
The speed of consensus across the industry is a huge factor. Despite the best efforts of EPCGlobal and other stakeholders, the industry has not defined standards as fast as some regulators would have liked. 2006 has been a year of solid progress for standards, however, enough uncertainty remains. This uncertainty has led to a Catch 22: investment will speed once clear standards are in place, which requires more extensive investments across the industry. For any system to work, these costs will need to be borne by all supply chain actors.
Technology Performance
ROI improves as technology costs drop a key driver of the tipping point. But, technology performance on a large scale remains a critical concern for both regulators and the industry. Confidence in technology is vitally important to driving further adoption, and continuing advancements and innovations are on the way. The security and visibility capabilities are central questions, but consumer and data privacy is becoming more important as deployment of RFID becomes more widespread.
Cost and performance must be properly balanced and aligned. There are real, bottom-line benefits to be gained from enhanced supply chain visibility and security. They include faster turns, reduced inventory costs, optimized partner relationships and reduced risk. However, most HLS organizations need to focus on operational nuts and bolts before they can start ROI projections. While there are many potential benefits to be realized from RFID adoption and supply chain adoption, business cases should be realistic about achieving them. And companies in the piloting stage should go into their programs with open eyes, normalized expectations and no pre-determined agendas. Thats the business reality.
Where We Go from Here
A range of interdependencies drives complexity in track-and-trace, which is a key factor slowing adoption. However, its not just a science project that can be put off indefinitely. Its a unique but urgent challenge. HLS organizations cant simply wait for technology advancements to solve the problem.
In our view, the most influential variables are in trade relations and collaborative process change. Those areas should be the focus for supply chain players and industry because they point the way to linking supply chain security to strategic business and IT initiatives and core enterprise objectives. Whats the bottom line? The issue of supply chain security isnt going away, nor will the pressing need to make significant investments in RFID and track-and-trace. The obstacles faced by the industry are daunting, but not insurmountable. Still, the industry has left itself considerable work to do to further increase supply chain security with these capabilities. The mantra is threefold: protect the patient, comply with regulations, and execute a plan to drive business value from track and trace and RFID investments.