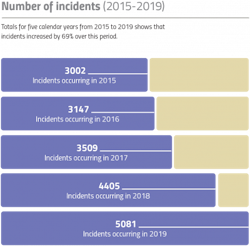
COVID-19 has changed our lives in many ways and exposed us to risks unconnected with the virus. One impact of the pandemic is a dramatic increase in the level of online drug sales, making products more vulnerable to counterfeiting and diversion.
One in four American consumers now buy their medicines online, and in 2018 the U.S. Food and Drug Administration estimated that only 3% of online pharmacies complied with U.S. pharmacy laws and practice standards. Writing in the American Journal of Tropical Medicine and Hygiene in 2019, doctors from the U.S. government, universities, hospitals, and the pharma company Pfizer warned that the rise in “falsified and substandard medicines” has become a “public health emergency” and that poor quality drugs exact an annual economic toll of up to $200 billion. On top of the direct harm they cause, counterfeit drugs are a major driver of antimicrobial resistance.
For criminals, counterfeiting is a low risk/high reward, with relatively lenient sentences and the potential to make millions of dollars in successful operations. The International Federation of Pharmaceutical Manufacturers and Associations reports that every medicine is vulnerable to counterfeiting, including life-saving medicines. The widespread use of pill presses means that we are now dealing with counterfeiters who can change production and ingredients rapidly to satisfy market demand. And while counterfeiters often target lifestyle drugs, life-saving medicines are the fastest-growing category.
There are many examples of what the rise in fake drugs means in human terms. Counterfeit fentanyl pills made to look like Xanax were discovered in every U.S. state in 2020 and linked to a sharp rise in fentanyl-related deaths. In the same year, Italian police seized 84 million Captagon pills that were produced by ISIS in Syria, containing amphetamines worth over $1 billion. The Drug Enforcement Administration’s National Drug Threat Assessment Report 2019 showed that although the supply of Chinese manufactured fentanyl had been restricted over the past decade, overall supplies were increasing from newly formed Mexican and Indian cartels.
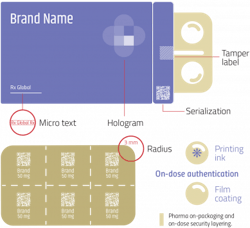
Pharmaceutical companies are accountable for ensuring that their medicine is safe when it comes into the hands of patients, and many countries have introduced serialization legislation that requires product identifiers to be affixed to each package to provide traceability throughout the distribution supply chain. Yet, despite the widespread introduction of serialization, the supply of counterfeit drugs is continuing to rise, and brand owners must develop robust strategies to safeguard consumers and protect their supply chain from external threats.
How the industry can fight back
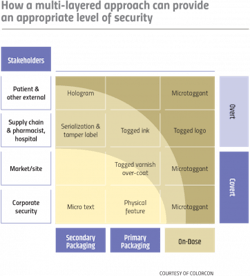
There is growing recognition that serialization alone will not solve the problem of counterfeit medicines. As serialization matures it will be valuable for track and trace, but for high value or high-risk products where supply chain security is imperative, more advanced measures are needed. Security technologies may be classified as overt or covert:
- Overt technologies are features that are visible to consumers and are incorporated into a product or its packaging. Holograms on the packaging and pearlescent pigments incorporated into tablet coatings are examples of overt technologies that are difficult to mimic, but such devices may give patients a false sense of security and provide little protection as counterfeiters become more sophisticated and are able to replicate these features.
- Covert technologies are designed to be difficult to identify and require testing to authenticate the product. If the security features are not easily seen or detected, then it will be very difficult for a counterfeiting organization to find and defeat these measures.
For the highest risk products, in addition to the use of security tags on packaging, on-dose microtaggants offer a much higher level of security, and allow each individual tablet or capsule to be authenticated.
A move towards on-dose authentication
Traceability and security measures focused on the packaging level may not be enough to protect patients. Even if a package is authentic, it may be impossible to determine whether the medicine inside is real or fake and whether it has been diverted.
To combat this, there is now a move towards “on-dose authentication.” This is achieved by incorporating microtaggants into coatings or inks used on pharmaceutical and nutraceutical products — and there are many exciting new options and applications on the horizon.
Microtaggants are uniquely encoded materials that are virtually impossible to replicate or reverse engineer. They can be incorporated into tablet coatings or into the inks used on tablets or capsules, and may then be detected using either field or lab-based equipment. This reduces the risk of counterfeiting and product diversion, and facilitates quality control and returns monitoring. Crucially the technology requires no additional manufacturing equipment or processes, which makes it economical and easy to implement. The FDA has stated that when microtaggants are pharmaceutically inactive and incorporated into new or existing drugs, they can be treated as excipients without the need for further clinical trials, allowing them to be incorporated into drugs already on the market, as well as into new drug products.
Two different types of microtaggants are available — one produced from non-biologic DNA and the other from silica. These taggants are not visible to the naked eye and are used in very small quantities but can easily be detected using field instruments.
DNA microtaggants
DNA is robust and easy to detect and, because it is possible to produce different versions of the same DNA molecule, it can be made regional, product, or company specific. The microtaggants are simply added to the standard tablet film coating or capsule printing process and can then be detected using appropriate reagents (like lock and key). The microtaggants are not damaged by exposure to heat and pressure during manufacturing, and the integrity of the DNA remains consistent throughout the shelf life of the product.
Silica microtaggants
Spectrally-encoded silica microtaggants can be detected by the way in which they reflect light, and can be customized with unique information for product verification and traceability. Like DNA, the microtaggants are incorporated into the film coating or printing ink and applied during the manufacturing process. Silica is already present in virtually all tablet and capsule formulations, making it an easy material to include.
Recent work has focused on developing convenient and reliable smartphone readers, and proofs of concept demonstrate that these could allow drugs with silica microtaggants to be tested directly in the field, allowing instant verification by law enforcement and border agencies.
The possibility of authentication apps would also allow patients to play larger roles in verifying their medication. Apps can also be leveraged to bring more value through patient engagement and brand loyalty. It also raises the possibility of incorporating microtaggants into drugs for clinical trials to ensure that the right product gets to the right patient at the right time, thus improving visibility and compliance and providing real-time patient support.
Choosing the best solution
It is important to understand how effective and reliable an authentication process will be, and the benefits that will be gained as a trade-off for the time and resources required to implement advanced technology. This should take account of costs, security, and capacity, including the ability to scale authentication points at required locations.
The cost per tablet of incorporating microtaggants is a fraction of a cent, and relative to other manufacturing costs the overall cost to the producer of the finished dosage form is negligible. If an authentication solution is machine-readable, it will be faster and more reliable than manual inspections, and suitable for high-volume applications. Quality teams which manage patient interactions will be able to use on-dose authentication to determine if a product is real or fake in their local offices, providing shorter turnaround times and more cost-effective processes by reducing the need for referrals to forensic labs, which are often overburdened.
Because the quantities of microtaggants that are added to the film coatings or inks are so small, the use of microtaggants does not affect how coatings are applied, and will not affect a product’s stability, disintegration or dissolution. It is worth noting that even though the use of microtaggants is not a safety issue, manufacturers must notify the regulator that the microtaggant is included as part of their annual report.
No one solution is perfect, and a multi-layered approach can incorporate the strengths of a range of security measures. It’s up to brand owners, product security, and risk officers to determine the most appropriate solution for their product — and at least now we can say that “we have the technology” to beat the scammers and take control through digital authentication.