There’s a lot that can go awry when pharma products reach the mixer — the wrong ingredients can be added, erroneous parameters can be set, or impurities can sneak into the equipment and contaminate the batch.
Ross Double Planetary Mixers consist of two identical blades that move in a planetary motion, rotating on their own axes as they orbit a common axis.
“Pharmaceutical products typically involve mixing a number of active and inactive ingredients together, yet demand a higher degree of homogeneity and quality control than many other types of products,” David Sieglitz, president, Readco Kurimoto, says. “The mixing step must achieve the required proportion of ingredients or the tablets won’t deliver the required efficacy.”
Mixing vendors have found ways to address many of these risks with updated tech that also helps manufacturers meet other goals such as using advanced software for data management, making sterility easier and more reliable, and reaching the holy grail for speed and efficiency — continuous processing.
Here’s a look at some of the latest innovations in mixing equipment.
Continuous manufacturing
“The increasing interest in continuous manufacturing for pharmaceutical products can be traced back to 2003 when the U.S. Food and Drug Administration issued draft guidance on process analytical technology (PAT),” says Rene Meira Medina, executive vice president, Gericke. “This led to regulatory changes in the U.S., EU and Japan that favor continuous processing as a method for producing quality products in a process that can be continuously measured.”
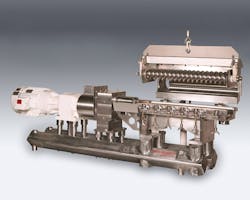
Gericke focused on this trend when developing its Continuous Manufacturing Modules (CMM), which turns raw materials into a finished product for tableting in five minutes without large storage tanks or batch mixers.
“We quickly found that short mixing times using gentle, low speeds with minimal drop heights not only met existing quality targets but actually offered improved quality,” Medina says. “As a PAT solution with integrated measurement and control systems, the CMM provided data documenting the improved quality, high formulation accuracy, and high speed of the continuous process.”
Readco Kurimoto has also innovated a line of continuous mixing processors that the company says can eliminate batch-to-batch variations while meeting tight quality requirements, and operating around the clock.
According to Siegletz, Readco’s
continuous processors use less space than batch processing, and save on energy costs.
“[The mixers also feature] an enclosed process, which eliminates or minimizes safety, cross contamination and dust hazards,” Siegletz says.
Readco sanitary continuous mixers automatically mix, encapsulate, crystallize and/or chemically react multiple powdered, liquid and/or viscous ingredients at the same time.
Customized controls
Although the basic technology behind mixing hasn’t changed in decades, today’s mixers can often be souped up with specific controls to suit each manufacturer’s needs,
automate the process, eliminate errors and collect useful data.
“The general trend is the interest in more advanced controls, custom software and user interfaces of pharmaceutical mixing equipment,” Christine Banaszek, sales manager, Charles Ross & Son Company, says. “Recipe controls and data management tools such as automated chart recorders have become essential to many pharmaceutical manufacturers.”
Ross recently produced a set of double planetary mixers catering to a customer-specific process with a level of technology and customization that the company says is “rarely seen in the industry at this size.” The mixers feature patented high viscosity blades, interchangeable jacketed vessels, electrohydraulic lift, recipe controls with data logger, and a dust-tight design.
Keeping it clean
“Cleanability is another major consideration,” Banaszek says.
To that end, Ross has focused on manufacturing mixers with an all stainless steel construction, smooth crevice-free hardware, sanitary tri-clamp connections, agitator designs that promote product containment within the mixing zone, high polish on wetted surfaces, CIP spray nozzles, and completely sealed gearboxes that all contribute to easier cleaning while reducing contamination risks.
Julia Schittko, the marketing manager at IKA, says the company has also innovated mixers with a stamp of approval from 3-A Sanitary Standards, an organization that provides third-party verification of sanitary levels.
“Requirements in the pharmaceutical industry have become more and more demanding,” Schittko says. “Which is why it’s important to have your equipment manufactured using guidelines such as the 3-A CIP (clean in place).”
IKA now offers a multiple stage homogenizer, a solid-liquid disperser, and high shear mixers, which are all 3-A CIP certified to ensure that the equipment is hygienic, and that all product surfaces can be mechanically cleaned or easily dismantled.
“Older equipment, which ‘only’ complies to certain criteria without a 3-A certificate, cannot be considered reliable for the required sanitary process,” Schittko says. “Industry experts are increasingly valuing the use of the official 3-A CIP claim.”
Whatever the needs of your facility, using updated mixing equipment with enhanced features can go a long way in making the mixing step clean, easy and error-free.