Blend Controls Boost Liquid Chromatography Performance
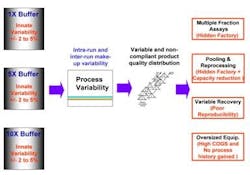
Traditional large scale pharmaceutical manufacturing operations use manual or fixed automation to prepare large volumes of reagent, storing them in large tanks. Such production-scale mixing of process fluids is much more difficult than at the bench-scale, where solutions and reagents typically are developed and optimized. Many individual mixing protocol steps, such as the addition of large volumes of water, can achieve no better than 2% accuracy. Measurement variability is another problem, reflecting variations in instrument resolution, sampling error, and intrinsic temperature and concentration gradation within tanks. Together, these factors can add another 2-3% to total process variability. Further, variability in raw material quality only increases total process variability, which, in turn is reflected in variable purity, potency, yield and recovery for final product. Such variability is a key contributor to many pharmaceutical companies' need for a "hidden factory," or an extensive non-value-adding infrastructure, to ensure product quality and safety (Figure 1).
Figure 1. Chromatography process variability starts with buffer or solution make-up; effects of that variability ripple through the entire process.Some manufacturers have used over-sized equipment, processing an entire batch as one lot to minimize variability within a manufacturing campaign. However, this increases the capital required for both equipment and liquid chromatography resin. It also increases risk, since the manufacturer must, in effect, put all of its eggs in one basket. It also requires larger, heavier equipment that is more difficult for operating staff to handle. Finally, this approach eliminates the opportunity for gaining improved process knowledge from multiple runs."Blending as Usual" Falls ShortConsider some representative automated blending approaches (Figures 2 and 3) that are widely used today. At first glance, both appear seductively simple and self-explanatory. However, neither approach addresses the variability inherent in concentrated reagent feedstocks today, which typically ranges from 2-5%.
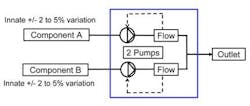
Figure 2. Blend control strategies that entail ratio control do little to overcome the variability inherent in reagent feedstocks.Using ratio control, the first approach shown in Figure 2, one would expect that extremely accurate (0.1%) mass flowmeters would permit equally accurate blending. This is not the case, however, because blending accuracy depends heavily upon where within the total flow range the equipment operates and what percentage blend composition is being made. Outside of a limited "sweet spot" within which blend composition still varies by 3-5%, variation worsens still.
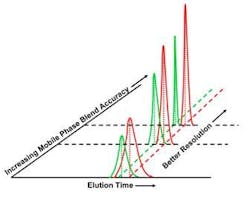
Figure 3.Use of a digital proportioning valve, likewise, does little to prevent variability.The second approach shown in Figure 3, using a digital proportioning valve, typically adds 4%-5% to feedstock variation. One manufacturer has developed an "upgrade" to help reduce this variability. This upgrade involved measuring the error profile and creating an equipment- and process-specific table of offsets. The unit's programmable controller was instructed to use these offsets to modify the target process value. Although this upgrade compensated for the systematic mechanical errors introduced by the blending configuration, it could not react to random equipment variations or differences in feedstock. In addition, developing the table is extremely labor intensive. Neither of these approaches provides adequate process control.Reagent Variability Reduced to 0.1%An alternative approach to reproducible blending was developed by TechniKrom several years ago. The approach trends blend accuracy and reproducibility as key process output variables (KPOVs), allowing users to determine the best control strategy along with critical control points. Bulk mixed feedstock compositions are treated as key process input variables (KPIVs). The method does not assume that KPIVs are accurate, and recognizes the futility of attempting to identify and control all potential sources of feedstock variability. Instead, the system is programmed so that blend is not released to the process until it meets preset specifications.The result is a programmable controller-based adaptive PAT system based on real-time feedback and adaptive control of the instantaneous composition of each blend as it is being created. Blend accuracy is assured, typically through real-time near infrared, ultraviolet or conductivity analysis, before being released to the process. Process-Scale ChromatographyInefficient, inaccurate and non-reproducible separations can be traced to the increase in variability of some KPIVs, as shown in Figure 4. In liquid-phase chromatography, even a small decrease in variability of the mobile phase can result in a large improvement in product resolution and reproducibility, which, in turn, dramatically affects product quality and profitability. Thus blend accuracy and reproducibility are both leveraged KPIVs and, hence, prime candidates for accurate process control.
Figure 4. The accuracy and precise composition of the mobile phase has a major impact on full-scale liquid chromatography separations.By applying adaptive PAT- based blending to the creation of 0.1% accurate and reproducible mobile phase blends, it is possible to transform the process as shown in Figure 4. Besides eliminating several "hidden factory" elements, such as the need for a tank farm, excessive quality control testing and pooling and rework of product fractions, the increased resolution and reduced process noise permits a much clearer view of the underpinning process that is so essential for improved process understanding and control.By programming the liquid chromatography system not to release mobile phase to the column until it is in spec, failure from a myriad of potential causes is prevented. Adaptive control thus enables the most precise and cost-effective resolution of product and prevents product loss, which, for biopharmaceuticals, can be considerable. Furthermore, using this system during process development can permit meaningful parametric optimization, seamless technology transfer and predictable process scale-up.The Bottom LineThe economic impact of using adaptive PAT has been quantified using actual manufacturing data from a high-value pharmaceutical product (Table I). The study used a conservative first-order estimate of the benefits of the improvements recognized by FMEA (failure mode and effect analysis). It found that using an isocratic or step-gradient approach to separate closely related species, rather than an accurate linear gradient, could easily reduce first-pass recovery to below 70%. Pooling and re-working the fractions containing the remaining 30%, assuming similar recovery efficiency, yielded a total of 91% recovery.In contrast, with adaptive PAT, first-pass recovery was 90% or higher. After reprocessing the side-cuts, total recovery increased to 99%. Reduction in variability translated into savings of $16 million per year.In this particular case, the assay samples taken throughout the manufacturing process represented a full 10% of the total drug produced. This $20 million loss of product represents yet another hidden cost of the traditional quality control-based approach to making drugs. With savings like these, it's clear that adaptive PAT controls make sound business sense. Table. Liquid Chromatography Cost Savings
|
Product Produced |
|
| 200g/year at $1 million per gram | $200 million |
| (25 runs of 8g per year) |
|
|
The |
|
| (Using popular inaccurate gradient model) |
|
| $250,000 |
| $100,000 |
| $100,000 |
| $75,000 |
| $500,000 |
| $18,000,000 |
| (70% recovery per run with 1 reprocessing results in 9% product loss) | |
With Adaptive PAT: Operational Cost Reduction
(With ideal accurate-gradient model)
- Labor (less 1 operator)
$75,000
- QC lab (reduce testing by 10x)
$90,000
- Reduced product consumption during QC
$450,000
(10 fractions reduced to 1 fraction)