Batch reporting is a critical component of the pharma manufacturing process because it provides the complete manufacturing history of a given product. This includes proof that good manufacturing practice was adhered to throughout production, and that all critical process parameters (CPPs) and critical quality attributes (CQAs) were met as prescribed in a drug’s regulatory filings.
To provide patients with the best drug quality possible, this comprehensive reporting procedure has traditionally required weeks to months following the completion of a batch. Once compiled, batch reports would often span hundreds of pages in length, each of which would require a quality review to ensure compliance and identify errors prior to a product’s release.
Data integrity, integration, contextualization, analytics and other qualities are essential for ensuring effective and efficient reporting, and ultimately for safeguarding the public — but existing procedures make it difficult to meet these and other requirements. By leveraging new analytics tools to create integrated and automated reports, pharma manufacturers can expedite the batch record generation and review process.
Batch report procedural shortcomings
Time is by far the greatest limitation in batch reporting procedures today, with manufacturers expected to meet strict production and distribution schedules. Because batch reporting can be cumbersome and time-consuming, it limits the time process engineers and data analysts have available to improve batching process efficiency, increase overall product throughput, and reduce production costs.
Batch reports are typically a manual collection of smaller reports, test results and investigations, each of which is used collectively to verify product safety and quality prior to its release to patients. The data making up these reports can span multiple platforms, such as manufacturing execution systems, process historians, laboratory information management systems, and computer maintenance management systems. Generating batch records and reviewing the reports are often very labor-intensive, even when using electronic batch records. While these records provide reduced post-process review latency, real-time review and monitoring capabilities are limited.
Despite the inclusive nature of batch reports, traditional reporting fails to take advantage of the significant opportunity to gain insights into processes, increase throughput, and shorten time to market. Instead, many pharma manufacturers today treat manually-assembled batch reports as standalone quality records, leaving unharnessed potential on the table.
A multi-national organization used Seeq, an advanced analytics application, to build reports, and to identify anomalies leveraging proprietary ML algorithms.
Leveraging integrated and automated reports
To enhance process efficiency, expedite reporting procedures and improve data centralization, pharma manufacturers are increasingly using advanced analytics applications to connect and examine their data in new and more efficient ways. These applications empower pharma manufacturers to automate and further integrate the assembly of batch reports, freeing up valuable time for engineering teams to extract process insights for improving overall production outcomes.
Equipped with live data connections, advanced analytics applications interconnect data in a central location from all the steps of a batch process, and they provide built-in visualization options, enabling subject matter experts (SMEs) to hone in on time periods of interest. This empowers multidisciplinary teams to collaborate and analyze processes using all available data in near-real-time.
In addition to automatically generating reports and real-time dashboards, which can be displayed graphically or tabularly, these applications provide rapid report filtering and exception-based reporting. These built-in analytics tools can be further leveraged to expedite the typically tedious review process for CPPs, CQAs, major deviations investigations, and subsequent root cause analyses (RCA).
These capabilities enable pharma manufacturers to leverage existing data streams, tools, and SMEs to create integrated batch reports, providing a quick return on investment by reducing batch release times and generating near real-time CPP metrics. Advanced analytics applications can even be used to deploy machine learning (ML) models using the data, and like other software applications leveraged throughout the pharma industry, it is CFR 21 Part 11 compliant with cybersecurity built in, including audit trails.
Case studies spotlight benefits
By leveraging tools within advanced analytics applications, SMEs can perform calculations to identify and prioritize areas for operational improvement. These activities include finding process bottlenecks, calculating overall equipment effectiveness metrics, performing cycle time analysis, identifying equipment and instrument drift, implementing predictive maintenance, and qualitatively tying all analyses to business needs and priorities.
This novel approach takes advantage of an organization’s existing data systems, including integrations with business intelligence (BI) platforms. Highly integrated batch reports reduce rework, and they provide additional value to the organization’s original investments in data historians, analytics and BI tools. Implementing fully integrated reports is the first step of the journey toward automating report generation and review processes, and it also provides further insights with data-rich reports.
The following case studies spotlight the benefits organizations can leverage by automating batch reports within advanced analytics applications.
Exception-based report and review
Working with integrated batch reports built on advanced analytics applications provides the additional benefit of automatically-generated, exception-based sub-reports. By configuring the application to call out noncompliant items, SMEs and process experts can quickly devote their attention to the associated data and information required to investigate further. A high-level overview within reports provides a quick status update across batches, including pre-generated trends and reports, along with other relevant information to pinpoint deviations.
This approach significantly reduces the required batch record review time because quality reviewers and the accompanying team are provided with a clear indication of where to focus their investigation to complete the report and release the associated batch. Sub-reports can be configured with varying granularity. For example, at a high level, a sub-report may only highlight items requiring review prior to closing out the record. At a more granular level, sub-reports may identify operating conditions, equipment sensors, and measurements showing signs of degradation, along with improvement opportunities. The latter can be achieved by integrating ML or multivariate models with the reports, and it can be leveraged to improve overall process performance and reduce future deviations.
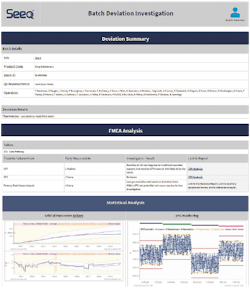
Although this example is not from the pharma industry, it is instructive because it shows how one company is currently leveraging an advanced analytics application to deploy exception-based reports. The example shows how a multi-national energy provider built reports and identified anomalies across hundreds of assets and thousands of signals by leveraging proprietary ML algorithms (Exhibit 1).
The associated automated exception-based reports were used to prioritize anomalies, and to identify top contributing signals outside of the CPP range that would have been previously ignored without this automated monitoring. The report workflow was used to identify deviations from advisory and quality limits, reducing multiple days of SME time for review to just a few hours of work.
Advanced analytics applications can automatically interconnect data and generate insights, empowering SMEs to rapidly identify root causes and corrective actions for batches.
Non-conformance investigations
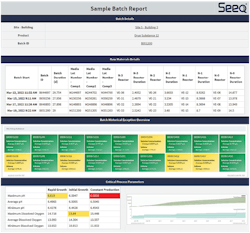
Non-conformances associated with batches are another common contributor to delayed releases, with RCA and the ensuing corrective and preventive actions sometimes delaying product release by weeks or months. Investigation times can be significantly reduced by using integrated batch reports, along with advanced analytics applications and historical batch data. By automatically generating insights and removing the lengthy manual data harvesting process, this type of software empowers SMEs to rapidly identify root causes and corrective actions (Exhibit 2).
Additional sub-reports can be configured using historical data, along with multivariate and machine learning models to pinpoint factors contributing to deviations, which can then be further analyzed. This ‘one-stop-shop’ for addressing atypical events associated with integrated batch reports can greatly increase throughput by reducing overall investigation times, leading to quicker batch release times and faster product delivery to patients.
A large biopharma manufacturer needed to integrate reporting into its RCA process to investigate an in-process drug substance lot that exceeded a CPP in the production bioreactor stage. The lot showed deteriorating conditions, with subsequent lots exhibiting similar trends.
Using pre-built reports and dashboards, the manufacturer was able to rapidly investigate the problem. SMEs identified the root cause and implemented a corrective action in a matter of hours by leveraging the integrated process historian, lab and equipment data. This allowed them to correct the deteriorating behavior, preventing future losses and saving the organization over $2 million.
Ensuring efficiency and quality
Efficient batch reporting is a critical step for accelerating a batch’s time to market, and by leveraging advanced analytics applications to create integrated and automated reports, pharma manufacturers can expedite the batch record generation and review process. Efficient batch reporting also empowers pharma manufacturers to unlock the full potential of batch reports by using their data to improve process efficiency, validate quality, increase throughput, maximize profits and guarantee reliable drug delivery to patients.