Plug and Play PAT, Anyone?
July 28, 2005
2 min read
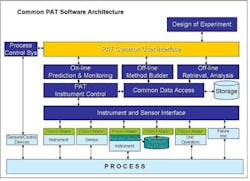
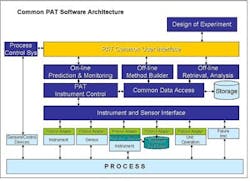
Widespread adoption of Process Analytical Technologies (PAT) will improve the way that pharmaceutical manufacturers design, monitor and control their processes, and allow them to predict process performance. PAT already enables this understanding. Unfortunately, monitoring, control and predictive capabilities are currently available only as individual components in different systems.One of the major issues limiting PAT today is the lack of open standards and interfaces between instrumentation and software. Each individual instrument vendor offers its own proprietary software, and, as a result, each PAT installation is unique. While we tolerated this situation when PAT was in its infancy, it has become a major problem, since PAT applications need to operate and acquire data from the process in real-time to monitor, control and optimize processes.As PAT applications proliferate, so are implementations with custom interfaces, point-to-point interfaces, custom modelers, and data islands. These are no longer acceptable. If PAT applications are to help in multi-factorial analysis, they must interoperate in control and modeling space.Given this need for an integrated environment, we propose that a Common PAT Software be developed, based on market and public standards. This would be commodity, off the shelf software featuring a standard user interface. This interface would be used to:
- set up and run analyzers
- provide a common modeling environment
- serve as a single environment in which to build and configure analyzers
- facilitate the archiving of PAT data in storage systems.
About the Author
Sign up for our eNewsletters
Get the latest news and updates