Multivariate Analysis for Manufacturing Quality Systems: Lessons from Novartis Tableting Operations
Sept. 30, 2005
7 min read
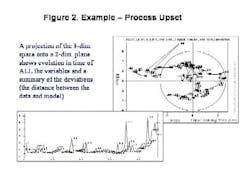
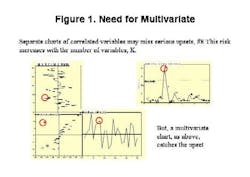
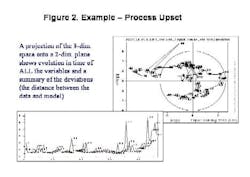
Final drug quality is influenced by everything that happens during manufacturing each process step, each ingredient, the condition of the equipment and even subtle changes in the manufacturing environment itself can lead to variations in product quality. The univariate specifications presently used for characterization of raw materials cannot adequately describe their quality or influence on the final product, often allowing problems to go undetected (Figure 1, below).Only by developing multivariate models that account for each potential cause of variability, and by applying process analytics, can drug manufacturers establish a foundation for manufacturing quality systems at their facilities. MVA should be a cornerstone of any PAT program.Unfortunately, for many people today, PAT is synonymous with process analytical chemistry, when its really much more. Process analytical chemistry may be a fast and precise pharmaceutical quality control tool, but, without modeling, it only shows a fraction of the variability picture and provides limited benefit. Analytics alone will not provide early fault detection or improve process knowledge, and may not align well with an organizations larger business strategy.Multivariate analysis (MVA; see "Demystifying Multivariate Analysis" for McCready's primer on this) allows manufacturers to model the influence of various processing steps and ingredients, so that major quality risk factors can be identified, even before a decision is made to invest in new analytical equipment.This article will show how combined MVA of raw materials and each production step is used to develop the foundation for a pharmaceutical manufacturing quality systems approach. Other industries, notably petrochemical, semiconductor and foods, have established such an approach, and pharmaceutical manufacturers are moving toward that still-elusive goal.The technology neednt require investments in new sensors, although it can point to a need for such investment, and provide insights required for smarter purchases.The article will also highlight some examples of what Novartis and Umetrics have learned so far from an ongoing project at the Swiss pharmaceutical manufacturers Suffern, N.Y. facility. At Suffern, MVA has been used to develop models, which have been calibrated and tested on an existing tableting production line, using existing sensors and raw material measurements. The resulting models are being used successfully to monitor quality in real time, detect faults, and predict final product quality. (Editors Note: For more information on this project, read November/Decembers issue of Pharmaceutical Manufacturing.)Why univariate analysis doesnt workUnivariate tools are often not adequate or affective for monitoring of pharma and biotech processes, which are complex and not well understood. Much of the important information in pharma production is contained in the correlation of the variables rather than the state of specific parameters. Multivariate tools model the relationships between variables, extracting more information from a process or spectra than univariate methods. The result is a greater sensitivity to subtle changes and less risk of missing serious upsets. Multivariate methods provide visual summaries of plant performance with tools for diagnosis of changes in operation. Consider one example of a pharmaceutical process upset (Figure 2, below); by projecting the 33-dimensional space onto a 2-dimensional plane, the model shows how the plant performance drifts in time.MVA not only provides more insight into raw material quality variances, but allows users to combine spectral and wet chemistry data, and to model the relationship between raw material and final product quality. It also allows users to address the complexities of batch monitoring, including:
- Time variance;
- Mixed data, including initial conditions, process data and final product quality data;
- The many unit operations, including granulation, drying, compression, and coating, that are responsible for final product quality.
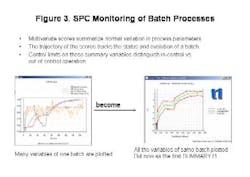
- Summarize normal variation in process parameters;
- Track the status and evolution of the batch;
- Allow users to distinguish between in- and out-of-control operations via control limits on summary variables (Figure 3, below).
About the Author
Chris McCready
Umetrics
Sign up for our eNewsletters
Get the latest news and updates