Using Raman to Track API and Solid Dosage Forms During Drug Development
Oct. 4, 2005
4 min read
Many pharmaceutical quality programs fail to account for the fact that API forms can change during drug development. They may be the same compound, chemically, but have different particle sizes or morphologies. In tableting or encapsulation, this fact can lead to processing problems for example, when an API particle that was spherical early on in the development process becomes rod-shaped later on, then causes agglomeration problems during blending.Worse yet, differences in form can also result in quality problems or adverse reactions in patients. This is especially important in crystallization, since different forms or polymorphs of the same compound may have vastly different properties different solubilities, dissolution rates, stabilities or bioavailabilities. As G. McGeorge has written, FDA expects that [any] crystallization process is in control and that the polymorph generated will provide predictable properties in-vivo. [1].Both NIR and Raman spectroscopy are being used to study and monitor API forms during development to understand, correct and improve processes, facilitate continuous processing and improve quality. This brief article will focus on Raman and its use in detecting crystal polymorphs during API development, as well as its use in tableting. It will summarize how the technology is being used, describe the benefits it offers and briefly compare its performance with NIR.Raman Now User-Friendly
Stimulating more interest in Raman spectroscopy is the fact that the systems have become much easier to operate over the years, and the technique offers such benefits as flexible sampling, including the possibility of remote sampling, and confocal optics. It also allows product to be sampled through containers, and can be used with various types of samples including liquids, slurries, pastes, solids and powders.The technique also allows users to better understand the chemistry of the process being studied, including not only crystallinity, but particle characteristics, reaction intermediates, reaction equilibria and timing optimization.It can be used to detect end points for a process that will result in optimal product quality, and is already being used for:
Stimulating more interest in Raman spectroscopy is the fact that the systems have become much easier to operate over the years, and the technique offers such benefits as flexible sampling, including the possibility of remote sampling, and confocal optics. It also allows product to be sampled through containers, and can be used with various types of samples including liquids, slurries, pastes, solids and powders.The technique also allows users to better understand the chemistry of the process being studied, including not only crystallinity, but particle characteristics, reaction intermediates, reaction equilibria and timing optimization.It can be used to detect end points for a process that will result in optimal product quality, and is already being used for:
- Structure elucidation
- Crystallization
- Polymorphism
- Solvate and hydrate forms
- Radiochemistry
- Reaction monitoring
- Grignard reactions
- Hydrogenations
- Nitrations
- Slurry monitoring
- Polymerization reactions
- Fermentations.
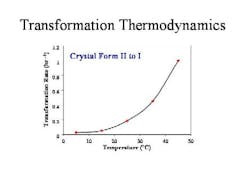
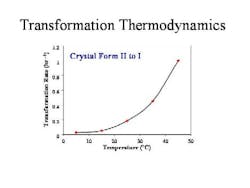
From these measurements (Figure 2, below), the thermodynamics of transformation could be summarized in a simple graph, and used to optimize process conditions.
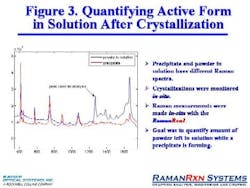
In another case involving the in-situ hydration of theophylline, Raman was able to determine the exact amount of active form left in the solution as precipitate formed (Figure 3, below).
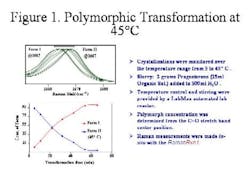
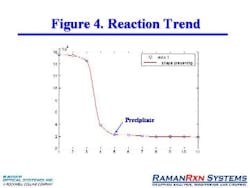
The phase transition was studied at a constant temperature and a calibration curve was developed, ultimately leading to a simple graph of the overall reaction trend, pinpointing the exact point at which precipitate was formed. (Figure 4, below).
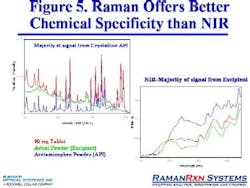
Raman for Solid Dosage FormsCurrently, most tablet analyses are done with HPLC, a specific and reproducible technique that nonetheless can be tedious to use, difficult to verify, and costly. Furthermore, using HPLC provides an incomplete picture. Applying Raman to tablet analysis offers significant benefits, as the resolved spectral density of he technique is generally very good. On the negative side, signal-to-noise can sometimes be an issue.Kaiser has now developed a PhAT system to address traditional Ramans limitations for solid state chemistry analyses. In the future, spectroscopic analysis of final solid dosage forms using techniques including Raman will offer the fastest and most complete way to assess product quality. However, users will need to increase their comfort level with applying chemometric techniques including MVA.Raman vs. NIR
The following chart compares both techniques:Dispersive RamanNIREmission
Absorption Fundamental information
Overtones No sample preparation needed
No sample preparation needed Process capable measurements
Process capable measurements High spectral density
Unresolved information Limited sensitivity to physical status
Sensitivity to particle size and compaction Representative sampling
Representative sampling Potentially very good LOQ
Good LOQ Smaller number of calibration samples
Large number of calibration samples Models potentially more robust and easier to develop
Models potentially less robust and complex
The following chart compares both techniques:Dispersive RamanNIR
ReferenceMcGeorge, G., Combining Raman Spectroscopy and Microscopy to Support Pharmaceutical Development, American Pharmaceutical Review, 1-4, 2003.
About the Author
David Strachan
Mark Kemper
Sign up for our eNewsletters
Get the latest news and updates