Consider a market where user safety and performance efficacy are of paramount importance. The product that fills the need is expensive, one-of-a-kind and, if it doesn’t arrive on time, is a catastrophic failure. Now imagine the factory: approved raw materials are blended in specialized vessels, formed into the final configuration and shipped. No final test. No confirmation that in-process control really worked.
Does that scenario sound like future shock for the pharmaceutical manufacturing industry? Well, the only tablets in this factory are headache remedies in the employee lounge. This is Bavaria Yachts, which produces over 2,000 ocean-going sailboats each year and never does a single final test.1
What happens if the product fails in use? Someone may drown. So what did it take for the manufacturer to have such absolute confidence in its mid-stream processes that it can skip end-of-the-line testing? Three critical components got Bavaria to that point: exhaustive review of process variability (and the attendant failure modes), management commitment to the program, and large capital expenditures.
“Bavaria has spent millions to automate production and contain labor costs,”2 says author Bill Springer.
And now to tablets as an end-product rather than an end-use palliative: Pharmaceutical manufacturing, pharma for short, doesn’t do nearly as well in the time-to-market consistency arena. Oh, sure, its products are safe and effective, but it scraps a lot of material on the way to the shipping dock. Why is that? The unpleasant answer is that pharma doesn’t pay attention to what can go wrong as it blends disparate materials into a single dosage unit.
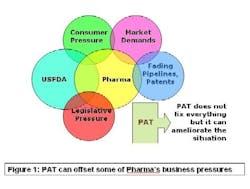
PAT — Process Analytical Technology — is supposed to fix that problem. As Figure 1 (below)indicates, lack of manufacturing efficiency isn’t the industry’s only problem, but it is an avenue that can ease some of the pressures on this beleaguered business. PAT is a drive to shoehorn pharma manufacturing into the efficiency footprint of the overall batch chemical industry.
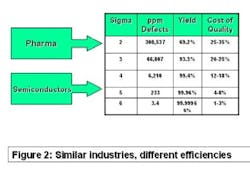
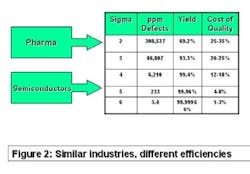
The best comparator may be the semiconductor industry. Chip makers produce items that have high unit cost, with no tolerance for product failure. They sweat time-to-market and time-on-market issues and face competitive pressure daily. Solid-state electronic devices are made of costly precursor chemicals using sophisticated production machinery. But as Figure 2 (below) shows, they experience less than four defective products per million manufactured. Pharma, on the other hand, may experience more than 100,000 in-process failures per million!
Why the difference? Chip makers understand every aspect of their processes and control them accordingly. Pharma pays less attention to the role of chemical ingredients in its processing machinery and depends concomitantly on end-of-the line testing. To be sure, this is a quandary mandated by law, but it’s a problem nevertheless.
In a flurry of enlightened self-interest, the FDA began an initiative to stimulate the pharma industry’s conversion to semiconductor or sailboat efficiency levels. You’re reading an article by a PAT pundit, in a newsletter devoted exclusively to PAT issues, so you’re probably well aware of the situation. Here are two questions, though. Is PAT taking hold, and how do you make it work?
FDA’s John Simmons says, “If you think of PAT as an isolated set of applications, I think that you are missing the point. The FDA would like PAT to become commonplace – not to be an initiative, but common practice.”3 Commonly accepted wisdom says that FDA has approved 12 PAT-based programs, and has a portfolio of 31 Letters of Intent. Obviously, the concept has taken hold, but what about making it work?
PAT meetings are replete with breast-beating, soul-searching and spats over issues such as calibration and standards. A newcomer would have to conclude that PAT is like the apocryphal elephant assessed by those wise blind men. In fact, it isn’t mystical or even unique. PAT is simply good manufacturing practice — note the lower-case letters! Bavaria Yachts doesn’t send representatives to PAT meetings, but they reap its conceptual benefits daily. Pharma can do that, too.
Pharma plants are regulated, sailboat factories are not. So initiation of a PAT program has to take that into account. Other than that, it’s just planning to ensure that production levels don’t decline, making the right in-process spending choices, co-opting the regulators and when ready, supplanting an outdated manufacturing method. We’re smart people, so most of that prescription is easy. It’s the spending that will take some work.
Assuming that the number of signers-on is right, 43 pharma makers are initiating PAT programs right now. We have to assume that they are in accord with FDA’s suggested guidelines: study your process, learn what makes it tick, find a way to test and control variability while the ingredients are in the processing machinery, and reduce it stepwise toward completion. And when you’re completely comfortable, file an amended NDA for what has been a successful product.
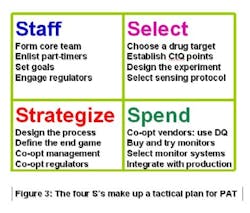
Mission statements are more easily assimilated when they are reduced to symbols. The four Ss in Figure 3 (below) can serve as the PAT Team’s aegis. The characters stand for Staff, Select, Strategize and Spend. When all of those actions happen, you’re ready for the fifth S: Supplant a GMP-tested product line with its parallel PAT counterpart. When T-T-M improves, then it signifies Success. Sounds like a sibilant snake, doesn’t it? Let’s get beyond the Ss and get on with making PAT work.
Staffing, or lack thereof, is one of the glaring missteps that foundered early PAT attempts. A PAT team needs a dedicated core, a cadre of senior-level professionals that will shepherd the program to completion. They can’t split their time or orientation while doing so. And they can’t do it by themselves. So, a successful PAT management group will use its core personnel for outreach to all affected operations within the company. Historically, PAT team leaders have come from the analytical ranks. They need expertise in production, engineering, finance and regulatory affairs.
Outreach and inclusion in the PAT mission will provide that. And they can’t ignore the regulators. Early dialogue with the FDA’s “Patriot”-trained PAT inspectors will pave the way toward that fifth S.
A team that selects a problematic drug product, or a candidate that is not yet marketed, for its initial PAT effort risks a shunt to an undesirable S: Shooting oneself in the foot. The best way to make PAT work is by modeling something that already works. Strategizing the right choice opens up the pathway to success: if it delivers measurable rewards to the company, then the effort was worthwhile.
Keep in mind that the ultimate PAT goal for your company is to make money. You can’t generate profits without spending; thus the fourth S.
PAT is good manufacturing practice — again lower case. Define “good” in GMP — upper case — terms and you’ll end up testing a non-optimal manufacturing process. A PAT program means continual and probably sequential testing of “new” mixtures that will eventually become drug products. Why “new”? Because GMP testing not only focuses on the final product, it also ignores the significant effects of excipient materials in the process.
Your PAT program will test for those effects, as it will for excursions outside the allowable limits that came out of your strategy planning. Using new “idiot savant” instruments and sensors in place of your “GMP-Genius” instruments is a virtual certainty. What will you use? You’ll have to select from a variety of outside-the-box approaches — a tactic that works but carries significant cost.
How will you make a compelling case to management – the same group that just swallowed increased personnel costs — for hardware expenditures, some of which may never integrate with your PAT production line? A vital aspect is opportunity cost – the recognition that opting for one course of action in an organization may preclude the opportunity to do something else. It’s quantifiable, in a way, but not for a PAT project bottom line.
Hundreds of PAT presentations tout ROI — Return on Investment — as a make-or-break metric for justifying a program. Use it at your peril unless you can use assumptions that are closely anchored in fact. All ROI planning requires assumptions, so it’s better to justify capital expenditures using NPV – Net Present Value, and IRR — Internal Rate of Return — fiscal arguments. These require assumptions, too, but because they are more rigorous, they resist challenge more effectively.
Here are some fatal flaws to avoid: Lack of flexibility, Impracticality, information overload and complexity.4
Wait a minute! Isn’t PAT supposed to be science-based? Why am I in the finance realm? Again, nothing can or will happen without cost vs. opportunity analysis and justification. Once you start planning from this vital benchmark, you’re more likely to hit the fifth S. There are tools to help you. It certainly isn’t the only resource, but a Microsoft Office-compatible program such as CaseMaker, from BusinessCase.com, includes all of the scenario-building and decision-making tools that you’ll need. Operate in the money sphere (oh, no, yet another “S”!) and the rest of the program will knit together in a successful launch.
References:
- Springer, Bill. Feeding the Machine, Sail Magazine, Boston, Mass., February 2006.
- Ibid.
- Simmons, John. Address at the 2005 Annual Meeting, American Association of Pharmaceutical Scientists, Nashville, Tenn., November 2005.
- www.businesscase.com, February 2006.
About the Author
John E. Carroll, C.Ph.C., is Managing Partner of Cadrai Technology Group. Cadrai Technology Group develops and presents focused training programs for issues germane to the current needs of the analytical instrument industry, especially regarding pharmaceutical applications.
Carroll
As Managing Partner for Cadrai LLC, Carroll modified and introduced ion mobility spectrometers for cleaning verification, an automated fiber optic/UV sensed dissolution apparatus for rapid pharmaceutical product screening and numerous Near-Infrared spectrometric systems for dedicated pharmaceutical applications. All products used proprietary technology of the principal clients.
Previously, Carroll was Pharmaceutical Business Unit Manager for Perstorp Analytical Instruments (now Foss). In that position, he built a global, sustainable business that placed over 8,000 NIR test instruments within the pharmaceutical manufacturing industry.
Carroll has a B.A.S. in Engineering Technology/Chemistry, a M.B.A. (c) in International Marketing, and is a Certified Pharmaceutical Consultant. He has written over 40 published papers and is the author of “The NIR Desk Reference” (Carroll, He & Landa) and “The Handbook of FTIR” (Carroll). He is also the editor of “IR-MS: High Sensitivity and Selectivity for Organic Analysis” (Mattson & Carroll). E-mail him at [email protected].