Crystallization is a key unit operation within the pharmaceutical industry, and over 80% of drug products involve at least one crystallization step. Yet, despite its obvious importance, crystallization is still a relatively poorly understood process. Typical problems associated with crystallization include unsuitable final particle size distribution, impurity issues (including incorrect polymorph) and poor or inconsistent yield. There are also stringent regulatory commitments to which all pharmaceutical companies must adhere to ensure that the crystallization process is robust and repeatable.
However, the crystallization step offers a unique opportunity to control the size, shape, purity and yield of the final crystal product. This can be greatly facilitated by measurement of important crystallization characteristics such as the crystal size and the liquid phase concentration over the course of a batch. Ideally, these measurements should be made in situ so as to avoid the inherent difficulties associated with sampling.
The U.S. Food and Drug Administration’s (FDA’s) process analytical technology (PAT) initiative has established a framework that encourages pharmaceutical manufacturers to use in situ tools to understand how their processes behave. PAT can be applied to design, analyze and control crystallization during processing by measuring critical quality and performance attributes. In this article, we summarize some of the work that we’re doing to apply PAT to crystallization (see Table below, following Case Study 1), and present a number of case studies to illustrate how these tools can be used to improve crystallization processes.
Undesirable particle size
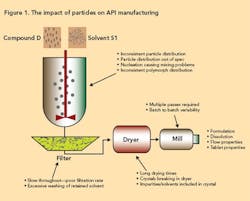
One of the biggest problems associated with crystallization is the formation of product with an undesirable particle size. Particle size affects such final product parameters as bioavailability and tablet strength. From a manufacturing viewpoint, poor particle size distribution can result in process bottlenecks, particularly during filtration and drying, leading to an inefficient process. Figure 1 (below) illustrates how particles can play a role in the manufacture of active pharmaceutical ingredient (API).
The final product particle size distribution is related to two key kinetic processes:
- nucleation of new crystals
- crystal growth.
By manipulating the relative magnitude of these mechanisms, it is possible to design a robust crystallization with a desirable final product particle size. For example, if small crystals are desired to increase bioavailability, or to avoid a milling step, nucleation should be favored over growth. But in a case where large crystals are desired — to reduce filtration time, for example — growth should be favored over nucleation. To understand how this can be achieved, it is vital to understand supersaturation, which drives nucleation and growth.
Supersaturation is defined as the difference between the actual solution concentration and the saturated solution concentration at a given temperature. It can be generated in many ways, including cooling, evaporation or anti-solvent addition. Cooling is the most common method used in the pharmaceutical industry.
Nucleation and growth rates are typically related to supersaturation according to Equations 1 and 2 (below). By controlling the supersaturation, one can control nucleation and growth rates and, thus, final particle size [1].
Where:
- B is nucleation rate (no./unit vol.)
- G is growth rate (length/unit time)
- kb and kg are kinetic nucleation and growth constants
- b and g are kinetic nucleation and growth orders
- ∆C is supersaturation (concentration units)
A simple strategy that can be used to increase the final product size is to maintain a low level of supersaturation throughout the batch. In this way, nucleation is minimized and growth will take place on the surface of existing crystals. Controlling the level of supersaturation can be achieved by employing a suitable cooling regime. This approach can also greatly enhance the final crystal size distribution. However, to do this accurately, knowledge of the solubility curve and metastable zone width (MSZW) is vital.
Case Study 1: Solubility curve and MSZW determination using FBRM [2]
Effective characterization of any crystallization starts with measuring the solubility curve and MSZW, which provides information on yield, suitable seeding locations and optimal zones of operation to obtain the desired particle size. FBRM can be used to measure the solubility curve and MSZW by accurately identifying the point of dissolution (point on the solubility curve) and point of nucleation (point on the MSZW) at various solute concentrations.
Here, an undersaturated solution is cooled at a fixed rate until the point of nucleation is measured with the FBRM, indicating a point on the MSZW. Next, the solution is heated slowly until the point of dissolution is measured with the FBRM indicating a point on the solubility curve. Solvent is then added to the system to change the concentration and the process is repeated. In this way, the solubility curve and MSZW can be measured rapidly over a wide range of temperatures. This process can be automated using an automated lab reactor (ALR) by introducing a feedback loop where the FBRM signal can be used to initiate the heating, cooling and dilution steps.
The MSZW will depend on a number of parameters, including the cooling rate, agitation intensity and the presence of solids. By assessing the impact of these parameters at the small scale, it is possible to identify potential scale-up issues. For example, if agitation intensity influences the MSZW in the lab, there is a good possibility there will be variability in the plant, where mixing tends to be poor.
Solubility and MSZW information allows suitable zones of operation to be identified. To encourage growth and minimize nucleation, crystallization should occur within the metastable zone. Seeding the process can help.
| Focused Beam Reflectance Measurement (FBRM) | In-situ probe that measures key properties relating to the size and shape of crystals in real time. Does not assume any shape, and is sensitive to fine particles |
| Particle Vision and Measurement (PVM) | In-situ probe-based video microscope that takes high quality images in real time. Used to complement FBRM data |
| Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) | In-situ probe used to measure liquid phase concentration and monitor supersaturation. In combination with FBRM and PVM, both the solid and liquid phase characteristics can be monitored |
| Computational Fluid Dynamics (CFD) | Allows mixing regime within a crystallizer to be characterized. Especially useful for modelling anti-solvent dispersion and for assessing feed point location |
| Microscopy | Despite sampling and preparation issues, an extremely simple and effective tool for characterizing crystal product |
Case Study 2: Using seeding, optimal cooling and FBRM to increase crystal size [3]
Seeding can be a useful method for increasing crystal size and ensuring a robust process. Seeding serves two important purposes. First, the addition of seed to a supersaturated solution allows the point of nucleation to be controlled, increasing the robustness of the process. Secondly, the addition of seed provides surface area for growth and minimizes nucleation.
A useful way to ensure that growth takes place is to isothermally age the seeds after they are added to the crystallizer. In this way, supersaturation can be fully consumed and the surface area available for further growth can be increased.
Cool slowly at first
The ideal cooling regime to employ after the isothermal age is to cool slowly at first when the surface area is small and then increase the cooling rate as the surface area of the crystals increases. This allows growth to be favored over the course of the whole batch while minimizing the batch time.
FBRM is ideal for tracking crystal dimension over time, and can be used to monitor the level of growth and nucleation. The FBRM data can be easily manipulated to focus on different crystal size ranges over time. This allows the level of crystal growth and nucleation to be monitored. FBRM can also be used to confirm that the seed has “taken,” and identify the end of the isothermal age where the FBRM trend flat-lines, indicating that growth is complete and all the supersaturation has been consumed.
While cooling is the most common method used to generate supersaturation, anti-solvent addition is also useful, especially where the temperature coefficient of solubility is low or where the final product is sensitive to temperature.
Case Study 3: Using FBRM, ATR-FTIR and PVM to optimize anti-solvent addition rates [4]
Anti-solvent addition typically generates supersaturation very rapidly and can be useful for the formation of small crystals or to enhance yield at the end of the batch. However, this rapid supersaturation generation rate can lead to irregularly shaped crystals and solvate/hydrate/polymorph issues. Carefully controlling the anti-solvent addition rate and, hence, the supersaturation generation rate, is vital to ensure a robust crystallization while guaranteeing a suitable particle size. The impact of addition rate can be assessed using FBRM to measure the particle size and number; PVM, to identify differences in shape and the degree of agglomeration and ATR-FTIR to monitor the prevailing level of supersaturation. In this way, the most suitable addition rate can be chosen.
Mixing
Another very important variable to consider for anti-solvent crystallization is mixing. Adequate dispersion of the anti-solvent into the solution is needed to avoid regions of locally high supersaturation near the addition point that can lead to premature nucleation and a non-robust process.
A simple technique to improve anti-solvent dispersion is to use a narrow anti-solvent addition pipe. The velocity of the anti-solvent impacting the liquid surface is increased, allowing better penetration of the anti-solvent into the bulk solution and thus improved mixing.
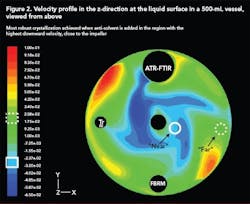
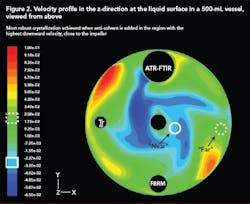
Case Study 4: Using CFD to scale up anti-solvent crystallization [5]
Scale-up of anti-solvent crystallization is made very difficult by the drastic difference in mixing capabilities at the lab and plant scales. CFD is a very useful tool that can be used to model mixing at both scales for any reactor and/or impeller geometry. In this way, suitable mixing conditions at the small scale can be identified and compared to conditions at the large scale.
Figure 2 (below) shows a velocity profile in the z-direction (upward and downward) at the surface of a 500-mL vessel generated using CFD. The effect of anti-solvent addition location on the MSZW was investigated experimentally and by combining this data with a CFD model, generated using a software package by Fluent (Lebanon, N.H.), the most suitable addition location was identified as the region where velocity in the downward direction was most intense. The high downward velocity allowed rapid incorporation of the anti-solvent into the solution and eliminated regions of high supersaturation close to the addition point.
With this in mind, similar velocity profiles were generated for a 70-L vessel with a different geometry and impeller configuration. The best addition point was identified using CFD and a robust crystallization that minimized locally high nucleation rates near the addition point.
In conclusion, the use of in-situ tools combined with novel technologies such as CFD and a sound understanding of crystallization fundamentals allow for better process understanding and hence improved process design, faster development and a more robust scale-up of crystallization processes. These techniques also apply to the PAT framework, which encourages the timely measurement of key variables along with improved understanding of the process.
Acknowledgements
The authors wish to acknowledge the support of the Irish Research Council for Science, Engineering and Technology, and the Irish Higher Education Authority’s Program for Research in Third-Level Institutions.
References
- Myerson, Allan S.
- Handbook of Industrial Crystallization. Butterworth Heinemann, 1993.
- Barrett, P. and B. Glennon.
- Characterizing the Metastable Zone Width and Solubility Curve Using Lasentec FBRM and PVM. Chemical Engineering Research and Design, Vol. 80, p. 799, 2002.
- Barrett et al.
- A Review of the Use of Process Analytical Technology for the Understanding and Optimization of Production Batch Crystallization Processes. Organic Process Research and Development, Vol. 9, p. 348, 2005.
- O’Grady, D. and B. Glennon.
- Use of in-situ Instrumentation to Characterize Anti-solvent Addition Crystallization. AIChE Annual Mtg., Cincinnati, 2005.
- O’Grady, D. et al.
- Scaleup of Anti-solvent Crystallization Using in-situ Tools and Computational Fluid Dynamics. World Congress on Particle Technology 5, AIChE Spring Mtg., Orlando, 2006.
About the Authors
Des O’Grady graduated from the School of Chemical and Bioprocess Engineering with a B.E. in Chemical Engineering in 2002. He is currently completing his Ph.D. in the same department; his research focuses on the use of in-situ techniques for the characterization and scale-up of anti-solvent crystallization.
Mark Barrett graduated from the School of Chemical and Bioprocess Engineering with a B.E. in Chemical Engineering in 2005 and is currently undertaking his Ph.D. in the same department. His research focuses on the combination of novel techniques such as computational fluid dynamics with experimental crystallization studies to improve crystallization scale-up.
Eoin Casey is a lecturer in the UCD School of Chemical and BioProcess Engineering. His research interests are in bioprocess modelling and biofilm engineering.
Brian Glennon, Ph.D., is a senior lecturer in the UCD School of Chemical and Bioprocess Engineering. His research interests are in the general area of chemical and biological process scale-up, with specific interest in crystallization of organic and biological materials.