Can Pharmas IT Keep Pace With Collaboration and Outsourcing?
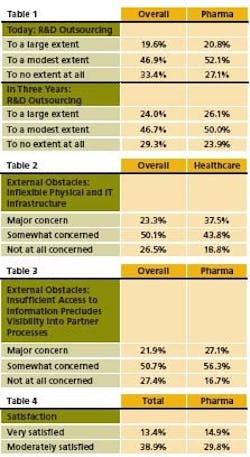
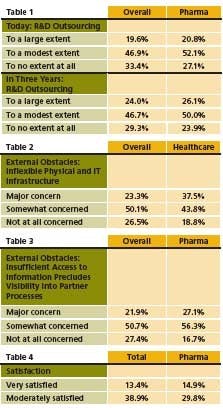
In July, SAP released results of a survey of over 353 C-level executives from diverse industries, 14% of them in pharma, life sciences and healthcare, which examined outsourcing and collaboration. Results suggest that outsourcing will continue to grow at a faster clip within pharma than in industry as a whole, moving at 20% per year versus 18% for all industries. Most of that growth will be seen in R&D (Table 1).
Respondents cite access to new skills, products and ideas as a key benefit of outsourcing, where almost six out of 10 pharma respondents cite access to new markets as one of the three most important drivers of collaboration. However, pharma respondents note concerns about inflexible IT, and note a need for increased transparency. Inflexible IT is a bigger issue in healthcare than in other sectors (Table 2).
The need for process visibility is another issue that separates pharma from other companies when it comes to collaboration (Table 3). As a result of above, pharma is much more likely to revamp IT and integrate processes to enhance visibility. Fewer C-level officials of pharma surveyed were satisfied with their IT infrastructure’s ability to support collaboration plans in the future (Table 4).
These findings suggest that pharma is facing some of the same economic arguments for agility in bringing products to market at a lower cost, says Jim Sabogal, VP of life sciences at SAP. “Increasingly, drug companies have to figure out how they can bring out products at far lower than this 800 million to 2 billion dollars per molecule, but targeted at a smaller segment of the patient population, and do that efficiently.”
“Now, other industries—consumer products, chemicals— are all used to collaborating with each other in terms of new manufacturing processes, new models, new ways of doing business. In life sciences and pharmaceuticals, it’s something they have to come to grips with,” Sabogal adds. “This whole notion of collaboration really extends throughout manufacturing,” Sabogal continues, “because, where they used to make a lot of money making key drugs, now it’s all about efficiency, throughput, how to negotiate contracts with partners, and, on top of all of this, the regulatory requirements.” Needs for product traceability and operational excellence are also driving changes in IT requirements, says Sabogal.