Take Advantage of Advanced Automation Technologies
Business continues to push for higher output, higher quality, greater consistency, more granular traceability, higher efficiencies and lower cost of goods manufactured — all while meeting regulatory compliance. In many companies, Plant, Corporate Engineering, IT and their partner integrators are pitching in to do their part to achieve success in meeting their business objectives.
The techies are helping production and compliance in many ways by using the most current technologies for computers, process controls, software, networking and security. These platforms are being integrated into comprehensive systems providing new levels of efficient and effective process controls, data capture and real-time information analysis.
Let’s explore the tools making automation in manufacturing contribute more to the bottom line than ever before. Note the following technologies and platforms are currently being used in validated environments by various manufacturers in the pharmaceutical and bio-pharmaceutical industries.
MAJOR SEGMENTS
An efficient manufacturing automation system makes optimal use of available components in five synchronized areas:
1. Process Controls Platform, such as PLC, PAC or DCS
2. HMI (Human Machine Interface)
3. Server Infrastructure
4. Historian and Relational Databases for Process Data and Events
5. Network and Access Security.
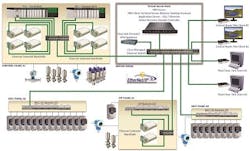
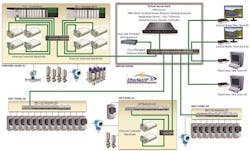
A typical manufacturing automation system using today’s advances in hardware and software platforms would look something like what’s depicted in Figure 1. Let’s examine each primary area of this validated automation system.
A typical manufacturing automation system using today’s advances in hardware and software platforms.
HEART AND SOUL
The heart and soul of any robust manufacturing automation system remains the Process Control platform. PLC-based systems have evolved to be at least on par with traditional DCS systems. Both control platforms offer features attractive to users from different perspectives and preferences. The common attributes of both platforms are:
• Ability to handle a variety of field devices found in today’s manufacturing environment.
• Ability to interface to multiple device communication networks such as EtherNet/IP, ControlNet, DeviceNet, Profibus, AS-i Bus, Foundation FieldBus.
• Programming structure based on ISA S-88 for Batch Processes.
• ISA-95 Model guidelines for the automated integration of enterprise and control systems.
• Closer integration with the HMIs and data historians, event archivers and relational databases.
Process controllers no longer live on the plant floor as separate “islands” of automation. Rather, they closely work together. Controllers are tightly integrated for bulk ingredient storage, compounding, purified water, CIP and SIP, finished product storage, even building automation systems. For high-availability and high-criticality applications, several process controller manufacturers offer redundant “hot-backup” configurations addressing the concerns over “single-point” hardware failure.
HMI
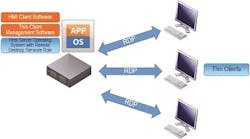
The user interface between the operator and the process, commonly referred to as the HMI (Human Machine Interface) continues to be mostly software-based graphics. The traditional HMI hardware platform was a Windows-based computer tied to either a proprietary bus or an Ethernet network. The preference in hardware platforms for HMIs is rapidly changing in manufacturing. Thin Clients and Terminal Servers are being used in both new applications and upgrades to legacy Process Control Systems. The diagram shown in Figure 2 reveals the typical configuration of a Thin Client/Terminal Services HMI application.
Typical configuration of a Thin Client/Terminal Services HMI application.
Thin Clients are diskless processors that interface over an Ethernet network to a server where HMI software and application files reside. Thin Client hardware requires minimal configuration compared to Thick Clients (traditional desktop computers). Many current implementations of Thin Clients use Microsoft Windows Terminal Services, now known as Remote Desktop Services (RDS), for connectivity. Using commercially available Thin Client management software on the host server allows for multiple, simultaneous HMI client sessions. So from one server, operators at different HMI workstations (Thin Clients) can independently and simultaneously view different process screens.
Plant operations have seen savings using Terminal Services and Thin Client technologies. Pharmaceutical manufacturers have also experienced:
• 33% savings in PC costs for operator workstations
• 55% reduction in power consumption by the operator workstations
• Reduction from four hours to less than one hour for a technician to replace an operator workstation.
SERVER INFRASTRUCTURE
Applications for HMI, S-88 batch processes, historians and relational databases are commonly run on server-class computers. Recent advances in optimizing the use of computer processing power have been made with the use of virtual machines, commonly referred to as virtualization. Software platforms are developed whereby multiple applications can be independently run on the same computer hardware platform. Separate operating systems are allowed to run simultaneously on the same computer hardware. Hardware virtualized servers remove the dependency of the hardware from the operating system and allow multiple, separate operating systems to share common hardware. Interfacing between operating systems and the physical server hardware is handled through the Hypervisor.
Virtualizing Computer Servers can significantly reduce the number of physical computing machines needed for a manufacturing automation system. Typical reductions in physical computers are six for a traditional installation to two physical virtualized computers giving the same computing power and performance. The computer hardware cost savings in this scenario is 66 percent. Electrical power consumption is also reduced by more than half in this scenario of using virtualized computer hardware.
DATA AND EVENTS HISTORIANS
Traditional methods for recording process parameters, such as Autoclave and SIP temperatures, to meet regulatory compliance relied on paper-based circular or strip chart recorders. Maintenance of these electro-mechanical devices was always a top priority to insure their reliability, accuracy and repeatability. Each chart recorder had multiple points of mechanical failure including the ink dispenser, ink supply and pen driver mechanisms.
Today, pharmaceutical and biopharmaceutical manufacturers are eliminating chart recorders and replacing them with historians; software-based data recorders extracting process parameter values on a continuous basis from the PLC, PAC or DCS process controllers. Today’s data historians can simultaneously and continuously record tens of thousands of individual process parameter points per second. Most data historians use data compression algorithms to optimize the use of mass storage either locally or in the cloud.
To meet regulatory compliance for data security, unalterable records and audit trail, historians must be validated to the stringent requirements outlined for electronic records in Title 21 CFR Part 11. Successful implementation of process data historians meeting Part 11 requirements begins at the Validation Master Plan for Computer System Validation (CSV). A systematic approach to the software development lifecycle greatly increases the probability of successful implementation of a software-based process data historian meeting regulatory compliance. Requirement specifications must clearly identify the detailed methodologies to implement Part 11. Then the validation protocols (IQ and OQ) must have detailed test scripts, with supporting documentation, verifying that all requirements for Part 11 electronic records are met. The net result is a reliable, robust and secure data historian that usurps the need for traditional chart recorders.
EVENT ARCHIVING
Traditional methods for recording events, such as batch start time and end time or target and actual ingredient quantities, have been paper records; MBRs or Manual Batch Records. MBRs do meet regulatory compliance and are very labor intensive. The MBR’s accuracy and completeness rely entirely on the operator. Maintaining paper MBRs becomes critical for traceability of the manufacturing process.
Like the historians described above, manufacturers are moving away from the paper-based MBRs and embracing software-based event archivers and report generators. Similar to the historians, event archivers, or transaction managers, record events germane to the manufacturing process. Typical recorded events include batch start and completion times, manual ingredient additions by the operator, target and actual ingredient fill quantities, operator interventions, and audit trail information. The significant advantage to using software-based transaction managers in lieu of MBRs is the critical event data is automatically captured via the process controllers and HMIs, then stored in a relational database such as Microsoft’s SQL. Once the event data is captured, batch and other production reports can be generated.
The event archiver and reports can also be created to be compliant with Part 11 for electronic records. The probability of success of implementing Part 11 compliant reports significantly increases when the integrator/programmer gets involved early in the system development lifecycle with those responsible for meeting regulatory requirements.
NETWORK SECURITY
Today’s requirements for process data, inter-device communications, and secure access to the process controls mandate the need to have the system design team give special attention to the networks; their physical installation and configuration to optimize data throughput and security. A variety of network types and topographies are used in manufacturing automation systems, but a Converged Plantwide Ethernet (CPwE) architecture including technologies such as EtherNet/IP and Device-Level Rings are becoming more prevalent for Industrial Automation and Control Systems (IACS) networks.
According to Cisco, “Modern, full-duplex, switched Ethernet networks offer real-time performance, including latency, jitter and packet-loss avoidance capabilities that meet or exceed the needs of IACS applications while offering better benefits than the older field-bus networks they replace. In addition, these modern networks have mature and tested technologies to safely secure the network and the systems they interconnect beyond what are available for the older field-bus networks.”¹
Critical to the stability of the physical network infrastructure are current and future networking requirements, knowledge of sound installation techniques, using certified network installers, following industry standards and performing cable verification after network installation. Cabling standards to consider are ANSI/TIA-1005 – M.I.C.E. and ANSI/TIA-569-C.0 (cable lengths).
Topics typically addressed during the specification of the network configuration include, and not necessarily limited to, consumer-grade versus commercial or industrial-grade network devices, managed versus unmanaged switches, and the use of VLAN networks.
The use of commercial or industrial-grade network devices is highly recommended. Most consumer grade devices, such as wireless access points and routers, do not have the range, throughput, or security features (RADIUS Authentication) of commercial or industrial grade devices. RADIUS stands for remote authentication dial in user service. Consumer-grade devices typically do not include the ability to implement VLANS or Virtual LAN segregation as do commercial or industrial grade devices.
The use of managed network switches is the preferred choice for many reasons. Managed switches have:
• Web-based interfaces to adjust port settings.
• The ability to set network speed (10 Mbps, 100 Mbps, 1 Gbps) and port duplex (half or full).
• Advanced switching technologies, such as Spanning Tree Protocol or IGMP Snooping (IGMP stands for Internet group management protocol).
• Spanning Tree Protocol (STP) providing path redundancy in the network.
• SNMP (Simple Network Management Protocol) built-in to remotely monitor switches on the network.
• Quality of Service (QoS) prioritizing critical traffic (such as video) within managed switches.
• The ability to create virtual local area networks (VLANs) or Virtual Networks. VLANs allow a switch to logically group devices together to isolate traffic between these groups even when the traffic is passing over the same physical switch.
Having the computers and process controllers on the same network affords another service being implemented in many manufacturing automation environments — secure VPN (Virtual Private Network) remote access. VPN access has become an extremely valuable tool for providing quick response by technicians and programmers who are not on-site, but who can connect to the systems when issues arise and resources at the plant are unable to resolve the problems before significant production losses occur. Many manufacturers have avoided losing hundreds of thousands of dollars of adulterated product by having the VPN access tunnel available to qualified and authorized technical personnel who can assist when response time is critical.
Pharmaceutical and biopharmaceutical manufacturers can improve their operations and efficiencies by effectively utilizing current technologies for process automation. These technologies have already been used in applications and have been validated to meet regulatory compliance. Critical to reaping the benefits of using these newer platforms is to have the system designers, production, maintenance, quality and compliance work closely together from the project’s inception to completion so all system requirements, specifications, regulatory requirements and validation documents are cohesive and uniform. Success is achievable for those willing to take advantage of the proven technology platforms available today.
Malisko Engineering Inc. is an integrator company helping clients enhance their productivity and profitability through manufacturing automation and a certified member of the Control System Integrators Association (CSIA), a global non-profit professional association that seeks to advance the industry of control system integration for the success of members and their clients.
REFERENCE
1 (Reference) - http://www.cisco.com/en/US/docs/solutions/Verticals/CPwE/CPwE_chapter1.html