True Process PLM: The Future of Pharma Manufacturing
More specifically, pharmaceutical manufacturers have not been able to realize the benefits of Product Lifecycle Management (PLM) technology, unlike their counterparts in the discrete manufacturing world. There, PLM is a technology-enabled discipline that has connected every step in the product lifecycle, enabling radical improvements in product quality and reliability while dramatically accelerating innovation.
Contrast that integrated approach with the current paradigm in pharmaceutical manufacturing, where information systems are largely fragmented and manual processes remain common. Fortunately, that is about to change. Converging factors are finally pushing drugmakers to adopt a more modern approach — one destined to improve efficiency, enhance quality and empower innovation like never before. As in the discrete world, technology-enabled PLM will again be the key.
The seeds of discrete PLM took root in the 1980s, when Computer-Aided Design technology revolutionized the way goods, such as cars and electronics, were developed and commercialized by providing a digital definition of the product. That not only unified the management of product knowledge, but also allowed the product design to become the central, digital structure driving all systems — most notably, in engineering and manufacturing. CAD and 3-D modeling now form the core elements of PLM in the world of discrete products.
Since discrete PLM is rooted in the 80s, it is somewhat ironic that drugmakers remain stuck in that very decade in many ways. The core technologies, concepts and approaches to managing pharmaceutical product knowledge and manufacturing continue to be dominated by thinking that evolved some 30+ years ago. Central to that thinking is the regulatory requirement for “documented evidence,” a product is being made according to an approved process. The key word there is “documented.” While paper-based approaches to manufacturing processes have largely been supplanted by electronic systems, the paradigm is still very much document-based.
• Labor-intensive (and thus error-prone) processes
• Lack of accountability and standardization
• Difficulty maintaining a “single version of the truth”
• Risk of knowledge loss from one manufacturing step to the next.
Perhaps worst of all, after a batch is manufactured, it is impossible to extract execution data in a way that can inform process optimization and business decisions.
In the past, some technology providers have claimed to offer PLM capabilities for process manufacturers. The problem there has been that drugs and other process-based products cannot be designed using CAD applications — the technology at the heart of discrete PLM. Digital design tools for process manufacturers have historically been standalone applications that cannot integrate with other systems the way CAD does. As a result, so-called PLM solutions for pharmaceutical manufacturing have continued to perpetuate the same disjointed, document-based paradigm.
Finally, however, pharmaceutical companies appear poised for a massive paradigm shift — powered by true PLM technology. There are two primary reasons this transformative change appears inevitable. First, a confluence of industry trends has rendered current approaches unsustainable. Second is the long-awaited emergence of CAD-like capabilities for pharmaceutical manufacturers.
Among the industry trends, the most significant is the dramatic rise in outsourcing to CMOs. Pharma companies accelerate time to market and reduce production costs through these partnerships, but lose visibility into the critical details of product manufacturing — sacrificing accountability and control over quality management. There is also the challenge of technical transfers, accompanied by the risk of knowledge loss.
Even internally, companies can have trouble retaining core product knowledge in these times of increasing employee turnover. Related to this concept, growth by acquisition helps to maintain information silos.
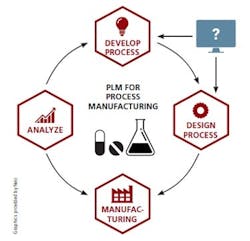
As these challenges mount, the foundational technology to enable true process PLM is finally emerging. At last, pharmaceutical manufacturers have their CAD equivalent — digital process design and simulation technology that integrates directly with existing systems including Manufacturing Execution Systems, Distributed Control Systems, data historians and even Enterprise Resource Planning systems.
True process PLM is now within reach, with the digital design serving as the central structure driving all systems. (I am referring to the product design in the discrete world and its analog in the process industries, the manufacturing process design.)
The digital process design can then become a powerful framework that connects all aspects of the product lifecycle, enabling drug manufacturers to:
Optimize production planning — Pharmaceutical companies will be able to easily compare the impact of different processes and production scenarios, while greater scheduling visibility enables CMOs to maximize asset utilization.
Automate the configuration of shop-floor systems — This will eliminate the inefficiency and risk associated with manual processes, and enable CMOs to more quickly begin manufacturing new products or change over from one product to another.
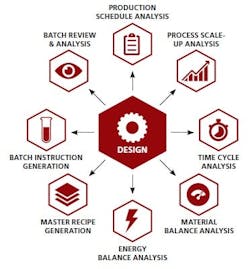
Enable fast, accurate production data analysis — Execution data can be automatically mapped back to the design, making it much easier to verify the recipe is being executed according to plan.
The end result: Timely data analysis will inform process improvements and other business decisions, creating a closed loop — the “holy grail” of manufacturing integration. At last, all stakeholders will be united around a “single version of the truth.” Seamless information sharing can streamline and accelerate manufacturing while enabling ongoing process and business optimization. Companies can improve both product quality and profit margins as a result. Ultimately, with scientists free to focus more on their core work, today’s document-based approach will give way to a new paradigm — focused on science and innovation rather than documentation.
Implementing a PLM solution seems daunting, but it doesn’t need to be. PLM technology ideally serves as the “glue” connecting different stages of the product lifecycle. With a modular solution, integration can occur in a phased manner with capabilities added incrementally until the manufacturing loop is closed:
1. It starts with a comprehensive digital process design tool that can communicate with your current systems.
2. You can then implement PLM technology that integrates with your digital design tool to provide a complete, end-to-end view of production scenarios for a product, enabling easy comparisons.
3. Production planning technology can be integrated with your design tool and production scenario browser, providing rich data to your ERP system and giving you a globally integrated platform for managing product supply.
4. You can now close the loop by deploying process analysis technology that integrates with both your shop-floor systems and design and planning software. The result: unprecedented insight to support critical business functions such as batch review and release and process optimization.