Navigating pharma’s leap into the predictive plant era
Within the confines of the pharmaceutical industry, Pharma 4.0 remains a laser-focused priority for many companies. Pharma 4.0, the International Society for Pharmaceutical Engineering's recommended pathways for pharma companies to embrace the future of technology and digitalization, is a critical roadmap for interested facilities that have yet to achieve their digital plant objectives fully.
As these companies navigate further down the road of digitalization, their plant processes become more optimized — in more ways than one — because they can obtain a gold mine of essential, actionable data.
While the advantages of embracing Pharma 4.0 and climbing the digitalization hierarchy are clear, scaling the ladder is easier said than done for some pharma companies. Companies may find it challenging to collect, correlate and utilize not just data but the data necessary to make a larger impact. The ability to obtain information and act in real time is a significant step from being in the digital silos and connected plant phases of their digitalization journey to the predictive plant stage.
Much of the correct data needed to transition into a predictive plant is derivative of real-time predictive analysis and an integrated plant network. This allows companies to use data-driven, proactive maintenance methods designed to analyze the condition of equipment and help predict when maintenance should be performed.
So, with data being so valuable and at companies' fingertips, understanding and implementing modern tools and technologies into processes could optimize and enhance key components like reliability, efficiency, safety and cost-effectiveness.
Digital plant maturity
Today, typically, there are five pillars to a company's digital plant maturity level:
- Predigital plant – Manual, paper-based process
- Digital silos – Some manual processes with islands of automation
- Connected plant – High level of automation with integration and system standardization
- Predictive plant – Integrated plant network with real-time predictive analysis
- Adaptive plant – Plant of the future that is autonomous, self-optimizing and plug and play
At present, most plants are stationed between the second and third pillars. Connected plants, third pillar, rely on regular and routine maintenance of equipment and assets to keep them running and prevent any costly unplanned downtime from unexpected equipment failure and potential safety hazards. However, this strategy requires planning and scheduling routine maintenance before a challenge or issue occurs. Companies would still need to be aware or consider that challenges can still happen after a routine checkup, which is why predictability is critical.
One of the main roadblocks plants face in transitioning into real-time predictive analysis and predictive plant era is picking the path that makes the most overall business sense. Many pharmaceutical businesses that have already received a predictive plant badge are discovering real-time predictive analysis:
- Increases reliability, productivity and asset life cycles
- Saves on maintenance and calibration costs
- Optimizes the calibration cycles based on data
- Minimizes unnecessary human error risks
- Reduces risk in between calibrations
- Offers flexibility for production schedules and equipment availability
Each benefit is essentially universal — business-wise — and ties back into risk mitigation and enhancing reliability, efficiency, safety and cost-effectiveness. The challenge for companies, again, is getting to a point where they can reap those benefits. It starts with the right instrumentation and technology.
Autonomous self-calibration in action
In the pharma sector, any inaccuracies result in potential safety concerns, lost time and monetary woes. Once a temperature sensor is determined to be inaccurate, mass-produced batches since the last calibration must be reevaluated and traced with a fine-toothed comb.
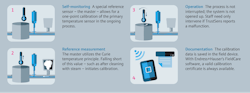
Regarding temperature instruments, specific devices contain predictive, self-calibrating sensors designed for hygienic applications — especially in an industry like pharma that requires strict compliance with GMP regulation. Self-calibrating sensors, along with inline verification, provide accurate measurements within the company’s specifications, and in the pharma industry, having confidence in accurate and valid data is essential.
Companies trust that devices work in a standard RTD process until an expert removes a device to calibrate or recalibrate it. With the self-calibrating sensor, the calibration process becomes more simplified and doesn't require removing the sensor from the process to perform a lab calibration. Ultimately, saving time and costs and keeping your process running.
These sensors autonomously trigger self-calibration as temperatures lower through a predefined threshold. Once the calibration is complete, there is an immediate pass-failure notification and calibration report generated. The calibration report is traceable to global standards and stored in the transmitter. This stored certificate can be downloaded as a PDF at the facility's convenience.
In one German facility with self-calibrating temperature sensors, a study was conducted for roughly one month — two batches and two calibrated performed daily. Eighty successful autonomous calibrations were performed, and the sensor accuracy fell within the specified cooling rate accuracy limits of -0.5 K/min and -16.5 K/min. The steam sterilizer's autonomous calibration point was within the desired sterilization process parameters at 118 degrees Celsius or 244.4 degrees Fahrenheit.
The pulse of processes with verification and condition monitoring technology
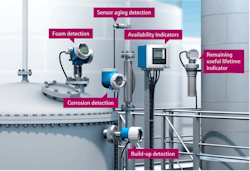
Using verification and monitoring technology in a connected environment turns data into knowledge. Seamless integration of field devices into all significant host systems and communication protocols enables reliable transmission of measured values and diagnostic data from the field to process control and asset management systems, improving predictive analysis approaches.
The pharma industry may rely on verification and condition monitoring technologies for greater predictability, most notably for the benefits between batch cycles. Verification enhances productivity alongside risk reduction because everything can be completed in situ and in real-time through regular calibration cycles, spreading cycles out and reducing the times they must be conducted.
In some pharma plants, verification technology has allowed personnel to compare data from multiple, consecutive verifications. Predictable trends are then detected and systematically tracked during the lifecycle of the measuring point, allowing for quick conclusions regarding the state of health or process-specific influences.
In other pharma plants, some modern verification technologies are bundled with monitoring functions and verification capabilities, providing even greater reliable insights into processes. With condition-based monitoring data, the presence of foam is detected, which is a substantial concern in specific pharmaceutical process applications as foam agents can be expensive.
Foam detection enhances reliability and cost efficiencies by reducing downtime in the plant and using costly anti-foam agents at unnecessary times. With the correct data provided by condition monitoring, plants can now determine the deployment of anti-foam agents. Foam detection also assists in reducing the amount of purifying that must happen to the product following the release of the anti-foam agent, as no unneeded amounts circulate, speeding up the purification process if they're completing it as a batch. Furthermore, there are foam detection sensors for both stainless and single use.
Single-use instruments for predictive analysis
Still relatively new, single-use instruments, specifically in Coriolis flowmeters, are showing signs of having a substantial impact in life science, pharma processes and predictive analysis.
While single-use Coriolis flowmeters are great for providing high accuracy flow measurement and multiple parameters such as mass flow, density and temperature, some are also able to provide in-line, real-time verifications and provide assurance the factory calibration is still sufficient. A barcode scanner pulls calibration information from the tubes calibrated at the factory in these single-use instruments. It then runs a verification process that includes checking all internal components and verifies that the meter is still working in the same condition as when it left the factory when calibrated.
In many ways, single-use technology ties back into the predictive analysis benefits of autonomous self-calibration. Once a new sensor is installed into that base unit, the transmitter can quickly verify and update specific settings automatically before starting a new batch shortly after.
Some pharma companies also have issues with calibration in closed, sterilized systems that have never broken the posterior boundary. In many cases, this causes those companies to refrain from calibration altogether. Calibration can be complex in certain situations because of the flowmeter's tube and liquid dependence. To properly calibrate, companies would need to send the tubes, with liquid intact, to a third party — a task most pharma companies may be hesitant to conduct. Knowing their processes are up-to-date and the ability to verify factory calibration information automatically is a game-changer for predictive analysis, again, improving a plant's process reliability and efficiency.
Certain single-use Coriolis flowmeters are designed to cater to single-use applications in the pharma industry. In the past, single-use applications were faced with accuracy and reliability concerns, but as market needs continue to change and adapt, single-use again became a topic of discussion. Today, single-use Coriolis flowmeters provide accurate and reliable measurements, critical in industries like life science where each move holds significant value.
Other understandable concerns for single-use Coriolis flowmeters centered on disposable manufacturing practices and increasing carbon footprints. Still, studies over the last ten years have shown that moving away from the traditional cleaning and sterilization process reduces a company's carbon footprint. Holistically, single-use technology can be considered a pivotal tool for production reliability, efficiency, cost-savings and sustainability.
The journey from the digital silos and connected plant phases to predictive plant may seem long and tedious, but with the correct data, instrumentation and technology, companies can take a stand against obsolescence and reach their digital maturation goals.
Reliability, efficiency, safety and cost-effectiveness can be enhanced with digital maturation. Thankfully, various tools and technologies help mitigate some of the challenges of leaping to becoming a predictive plant.
About the Author
Bethany Silva
Life Science Industry Manager, Endress+Hauser
Bethany Silva is the Life Sciences Industry Manager for Endress+Hauser, a globally operating process and laboratory instrumentation and automation supplier. In this capacity, she is responsible for the strategic direction, vision and growth of the Life Sciences Industry in the U.S. Bethany has worked with several life science companies with positions in research and development, process development, marketing and technical sales. She brings more than 20 years of experience in sales and marketing to the role, 13 years dedicated to life sciences.