FDA could run out of funds for drug approvals in five weeks
As the government shutdown drags on, the clock is ticking on the FDA’s ability to process drug approval applications.
According to a post on Twitter by FDA Commissioner Scott Gottlieb, the agency will run out of money for drug approvals in about five weeks.
Earlier this week, the agency asked about 400 employees to return to work — without pay — to focus on inspections of high-risk medical device, drug and biological manufacturing facilities.
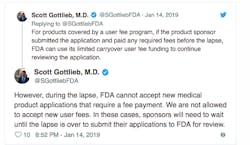
To keep the drug review process moving, Gottlieb said the agency has stretched its remaining funds from user fees paid by drug companies.
“We’ve stretched carryover drug user fees to get a longer runway should the shutdown continue, by for example sharply reducing any overhead charges to CDER/CBER. The slower burn rate gives us about five weeks left as of this week. The number could change,” Gottlieb posted on Monday.
The FDA cannot accept any new applications or collect fees until the shutdown is over.
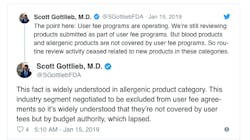
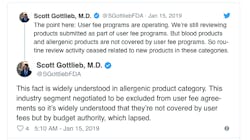
Gottlieb also Tweeted this week that the review of blood and allergenic products has ceased all together because those applications are not covered by user fees but instead fall under the budget authority. Aimmune Therapeutics, reported this week that it was told by the agency that its application for a peanut allergy medication cannot be reviewed at all until the shutdown ends.
An analyst told CNN that once the shutdown is over, the FDA will mostly likely have a backlog of drug applications that could slow down the review process for an unknown period of time.
The government shutdown started on Dec. 22. At the moment, negotiations between Congress and the Trump administration over the budget have stalled.
Read the full CNN report.
In the process of working with the FDA? Read more about what pharma companies need to know about work at the agency during the shutdown.