In recent years, Life Science companies have faced increased scrutiny on quality and operational risks, in particular supply chain risk management. Headlines are filled with examples of failures caused by ineffective management of these risks. In 2010, two major events highlighted the criticality of effective supply chain risk management. In May, headlines were filled with the theft of prescription drugs from a Connecticut warehouse. In a coordinated attack during the cover of night, thieves disabled a burglar alarm in the warehouse and carted away dozens of pallets loaded with antidepressants and anti-psychotic drugs.
In April, the Icelandic volcano eruption wreaked havoc on passenger airline travel and trade across Europe. Many Life Science companies were unprepared to handle the closure of major airports across Europe and were left scrambling to develop alternative transportation and shipping methods. In response, organizations needed to quickly develop and implement contingency plans to mitigate compliance risks, minimize financial impact and continue operations. Overall, quick and coordinated responses helped minimize the potential impact of this natural disaster, but longer disruptions, in some case only a matter of weeks, would have had significant impacts on operations. These events clearly highlighted how fragile today’s life science’s supply chains are.
Emergence of Virtual Supply Chains
The risks noted above are amplified in organizations with virtual operations. Outsourced supply chain operations have become a central part of many Life Science companies’ operational strategies. Today, many organizations are opting to outsource non-core competencies like distribution and logistics to specialized service providers. Additionally, the emergence of India and China as adequate suppliers of raw materials and critical drug product intermediates (e.g. Drug Substance or API) has accelerated the global sourcing trend. Virtual supply chains have proven to be an effective manner to build infrastructure quickly, lower operating costs and increase profits. This trend will certainly continue in the future.
Despite the advantages of virtual supply chains, there are inherent dangers associated with them that are driving the need to better manage suppliers, most notably:
• Heightened regulatory focus (e.g. ICH Q9) on managing risk—both internally and externally with partners/suppliers
• Life Science organizations are ultimately responsible for quality and regulatory compliance of their products
• Uniqueness of materials that may limit supplier options
• Global economic crisis impacting supplier solvency or ability to obtain credit/financing
• Suppliers lacking established risk management programs or tools to mitigate risks within their supply network
With the stakes increasing every day, Life Science organizations must become effective managers of the risks inherent in their outsourced supply chains.
Current Approach to Supplier Risk Management
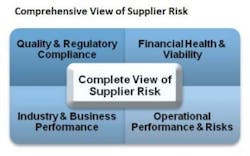
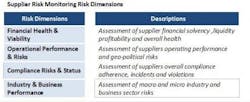
In response to the virtualization of supply chains, many Life Science organizations have developed supplier audit programs as a means to qualify or monitor ongoing supplier compliance. These programs have defined procedures that are typically executed by quality or procurement personnel and are geared towards uncovering compliance risks. In many instances, these programs are very effective in performing their intended purpose, which is determining compliance risks. However, typical supplier audit programs fall short in providing indications of the overall health and viability of a supplier and have inherent downfalls, most prominently:
- Narrow focus results in an incomplete assessment of overall supplier risk—lack of visibility into supplier’s business, operational and financial risks
- Reactive risk management approach—events or incident as triggers to perform supplier audits vs. continuous monitoring to pro-actively identifying risks before occurrence
- Processes and tools for assessing and prioritizing risk typically being subjective—non-programmatic or standardized approach to customizing and applying risks management tools within Life Sciences