Imagine a world where a patient’s records capture the brand, dosage and lot number of each drug and medical device she uses, along with the name of the physician who ordered the product and the nurse who administered it; where bedside scanning confirms that she gets the right product in the right dosage at the right time; where hospitals and pharmacies know the exact location of short-supply medical devices and drugs and when they can be delivered; where regulators can recall adulterated products with accuracy and speed from every point in the supply chain; and where manufacturers can monitor real-time demand changes and shift their production schedules accordingly.
In this world, patients would enjoy consistently safer and more effective healthcare, with fewer mistakes and shorter average hospital stays. Redundant activities and costs would be driven out of the system — reducing the cost of healthcare to society and enabling broader global patient access to cutting-edge medical technologies. Opportunities for innovation would open up — enabling new progress in personalized medicine, customized devices and mobile health.
This world is technologically possible today. But the reality is a long way from the ideal. Major pain points in the current healthcare value chain range from patient outcomes to supply chain efficiency, including the prevalence of medication errors, inefficient and ineffective product recalls and bloated inventories. The healthcare supply chain, from manufacturer to patient, remains fragmented, with limited visibility and interconnection. Certain channel partners are making progress by collaborating, and individual companies and even countries are documenting excellent results with cutting-edge practices. But only a few players are making these innovations and advances.
To build a world of interconnected, cost-effective healthcare, the healthcare industry could align around a single set of global standards that support the processes and capabilities required to achieve the kinds of benefits outlined above. The consumer and retail industries have demonstrated the value of this kind of standards alignment with their adoption of GS1 standard barcoding, which has reshaped these industries and created billions of dollars in value. While new processes, tools and systems were required to deliver this value, use of one single global standard was a critical prerequisite.
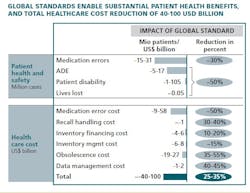
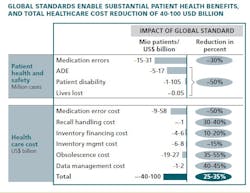
This year, McKinsey & Company carried out a research effort, with the participation of more than 80 healthcare industry and regulatory leaders around the world, to estimate the potential value of adopting a single global standard in healthcare. Taking a conservative approach, our models suggest that cost could be reduced by $40 - $100 billion globally (see exhibit), mainly from reduced follow-on cost of medication errors ($10 - $60 billion), cost from improved inventory management (financing, processing, obsolescence cost reduction of $30 - $40 billion), and reduced data management cost ($1 - $2 billion). The final figure may even be substantially higher, because global standards may deliver a variety of smaller benefits that are more uncertain or difficult to quantify.
Those are compelling numbers, but to make standards work, individual players in the healthcare value chain must have the confidence that the direct rewards will be worth the necessary investment. In this article, we will focus specifically on the costs and benefits of adopting such standards for pharmaceutical manufacturers.
The Business Case
For our models, we took the case of a representative global pharma manufacturer, with 25 packaging lines, annual revenue of $4 billion, and earnings before taxes of $720 million, (18% of sales, in line with McKinsey industry benchmarks). We assume 70% of revenue is earned in developed markets and 30% in developing markets (used to estimate exposure to high-counterfeit markets).
The key elements of global standards are product identification, location identification, and master data synchronization. Manufacturers would be required to make investments to implement these standards. The precise size of that investment depends on many factors, including the type of identification adopted (basic product identification including batch number and expiry date information, or separate serialized identification of each individual unit), whether barcoding is applied to primary or secondary packaging, and the state of the manufacturer’s current IT infrastructure to be extended to accommodate global standards. Our analysis suggests that such costs would range from $150,000 to $500,000 per packaging line and between $1 million and $5 million in licenses and software integration costs for the organization for our representative manufacturer.
Do the potential benefits justify the cost? We believe so. By adopting global standards in partnership with its trading partners, our representative pharmaceutical manufacturer might expect a range of benefits worth about $40 - $60 million annually, which represents about 1-2% of base revenue and about 5-10% in earnings before taxes. These benefits arise from a variety of sources, including reduced obsolescence, lower data management costs and improved transaction accuracy, and potentially a reduction in sales lost to counterfeits. In addition, a one-time cash flow benefit of about $90 million would accrue due to reduction in inventory assets, enabled by improved supply chain visibility. Accumulating the benefits and both one-time and annual costs over 10 years, we expect barcoding at the secondary packaging level to deliver about 20-25 times more benefits vs. costs. Serialization would have approximately a 4X benefit/cost ratio.
Beyond these significant financial advantages, standardization has the potential to greatly simplify and streamline many aspects of operations and customer interactions for pharma companies. Automatic master data synchronization would greatly reduce ad hoc customer requests for product information, decreasing the burden on manufacturer staff to respond to these requests and allowing them to spend more time on value-added customer service.
Generating reports could also become more efficient. Global manufacturers face significant challenges in rolling up data across divisions and regions. One executive told us that his finance, local and hub planning locations and various other functional units could create as many as five identification numbers for a single product within the same company. Leading organizations could potentially generate valuable insights from data faster than their competitors and gain competitive advantages in an increasingly data-driven world.
Finally, there is potential for manufacturers to contribute greatly to patient safety through adoption of global standards in three areas:
1. Raising the bar for counterfeiters by serializing products, to allow authentication at dispensing and usage points
2. Faster, more efficient recalls by capturing standard location identification and batch number information, and tracking how products move through the supply chain
3. Reduction of adverse drug events due to medication errors in hospitals by placing standardized barcoding at the primary packaging level so that bedside scanning can ensure the Five Rights.
Getting From Here to There
In the 1970s, the grocery industry formed a committee of leaders of major manufacturers and retailers. In consumer packaged goods, a few global players worked together tirelessly to align on GS1’s single global standard for the industry. More recently, the Consumer Goods Forum organized senior executives to define requirements for global data synchronization. These leaders worked together across the value chain, and their decisions drove adoption throughout the sector.
Healthcare industry leaders who are convinced of the benefits of global standards are in a position to work across competitive and customer-supplier relationship boundaries to agree on a common vision and approach. Customers, vendors, competitors and regulators will have to act and collaborate in new ways. Their aim will be to create interoperable systems; these are the enablers for change.
However, healthcare is a more fragmented and regional industry. Unlike consumer packaged goods, in healthcare the manufacturers are the largest and most global players, and can therefore play a unique role in driving adoption of a single global standard. On the other hand, they will bear significant incremental costs if, instead of a single global standard, requirements proliferate across customers and countries. The cost of managing the resulting complexity in packaging operations and distribution centers is significant — particularly considering the indirect costs of maintaining quality and compliance requirements. McKinsey estimates that if manufacturers are required to comply with two standards, instead of just one global standard, investments could increase by 15 - 25% (additional equipment and implementation) and operating expenses (conversion cost) by 5% (shorter production runs, more or longer changeovers, cost of supplies).
Manufacturers could realize significant benefits if they work together to shape processes, industry norms, channel partner agreements and data management responsibilities to create greater visibility to their products’ end-to-end supply chain and demand patterns. In retail, manufacturers benefited from access to point-of-sale data about shelf space, stock and retail forecasts, which enabled a second wave of supply chain optimization, including optimized assortments and delivery frequencies, collaborative forecasting and replenishment, and improved on-shelf availability. Healthcare manufacturers could also benefit greatly if they improve control over their products’ shipment and usage conditions, protect brand reputation and improve patient safety and effectiveness outcomes.
The healthcare industry is at a crossroads, and our research suggests that the case for alignment on a single global standard is compelling at both the total industry level, and for individual companies. More importantly, the case is compelling in terms of numbers of lives saved and medication errors averted. Industry leaders could seize the moment to create a true win-win opportunity, both for the industry and for patients.
Editor’s note: A complete description of the models, costs and benefits associated with global standards can be found in the white paper “Strength in Unity – The Case for Global Supply Chain Standards,” at www. mckinsey.com, under Client service/Operations/Latest thinking.
Published in the March 2013 issue of Pharmaceutical Manufacturing magazine
About the Author
Thomas Ebel
Katy George
Sign up for our eNewsletters
Get the latest news and updates