When it comes to preparing drugs and therapeutic treatments for market, assessing drug safety and efficacy is a complex and multi-layered process. Drug substances and products can be subjected to many environmental influences as they begin their journeys from the laboratory to the patient. Factors such as temperature, humidity, light and pH can have significant impacts on drug safety, efficacy and quality.
For this reason, testing is required to obtain a “stability profile,” providing proof that a drug will maintain its desired characteristics through manufacture, transport and supply, until consumption. Since climate zones can vary greatly and manual testing in each area takes considerable time and effort, more manufacturers are choosing to emulate multiple climate zones by completing stress tests in accelerated and extreme conditions.
Knowing how and when to complete forced degradation assessments to stress test drugs will help create comprehensive stability profiles.
What is drug stability testing?
Drug stability testing is a critical stage in drug production. It must take place before a drug reaches the market, and the process must comply with the International Council for the Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) sections Q1A-Q1F. Sections Q1A and Q1B refer specifically to stability and photostability. And, it is through these regulations that manufacturers can help to assure regulatory bodies and prescribers that drugs are safe and efficacious, and will perform in the way in which they are intended. In fact, key regulatory bodies, including the U.S. Food and Drug Administration (FDA), the European Commission (EC) and Health Canada, all recognize ICH requirements as part of their approval processes.
By exposing the drug to extreme conditions, the intrinsic stability of the drug can be assessed. Degradation pathways can generate byproducts that destabilize products and alter their performance, but the stability profile can help set parameters around shelf life and storage conditions to ensure safety and efficacy within these buffers. In turn, packaging solutions can be designed to protect the potency and purity of the product, while extending the storage time in which the product can be safely used.
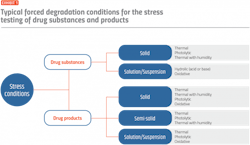
Four main environmental conditions have the greatest effect on a drug’s stability: temperature, humidity, light and pH. Temperature is one of the most critical conditions as physiochemical stability is only ideal within a narrow range of temperatures and an increase of 10°C can accelerate hydrolytic degradation by up to 500 percent.
The second condition, humidity, is a key factor in assessing both the degradants and active chemicals in a finished product. For this reason, the ICH recommends stress testing drugs at 90 percent humidity for a one-week period.
The third condition, light, can have a large impact on the chemical stability of a photosensitive drug, resulting in phototoxicity, photoallergy and photosensitization. Carefully controlling light in stability testing helps define these parameters and the necessary packaging required to protect the drug product and patient.
The fourth and final condition, pH, affects the stability of drugs that are prone to hydrolytic degradation, much like temperature.
All of these important parameters can now be tested in carefully controlled environments with precise programming used to replicate different climate zones in accelerated, extreme conditions.
Testing to the limits with forced degradation
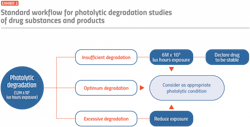
Many manufacturers are now using environmental or light chamber technology to control forced degradation and accurately define drug stability profiles. By using these self-contained testing environments, researchers can achieve extreme conditions quickly and replicate multiple climate zones in one location. Various stress conditions can be generated, depending on the type of drug substance or product (see Exhibit 1), and workflows can be established to set the parameters under which a drug can be defined as photostable (see Exhibit 2).
Environmental chamber technologies enable wet and dry testing to extremes, or incremental testing from excessively hot to very cold conditions. This is critical when testing thermolabile compounds, such as vitamins or peptides, where stricter parameters may need to be established to ensure stability.
Light chamber technologies take testing to the next level, combining temperature and humidity testing with additional light tests in the visible and ultraviolet spectrums. By assessing the physiochemical effect on a drug, processes such as oxidative photolysis, isomerization, dimerization, cyclization, rearrangements, decarboxylation and the hemolytic cleavage of various bonds can be understood in relation to active ingredients and degradation compounds.
Choosing your chamber
Environmental and light chamber technology can vary widely between manufacturers. Whether the technology will be used in research and development laboratories, or as part of quality control procedures, particular features will determine the usefulness and day-to-day practicalities of the system.
The temperature range offered by the chamber should ideally fall between 0°C and 60°C, with complete temperature uniformity delivered throughout the unit. When it comes to drugs that are thermolabile in nature, the chamber should be capable of allowing for thermal stress testing even more strenuous than the recommended ICH Q1A accelerated testing conditions.
Features such as directed airflow systems can also help standardize the environment by minimizing product desiccation. Humidity control is a critical factor and although the ICH stipulates humidity testing at 90 percent, it is good practice to work in conditions from ambient to 95 percent, and environmental chambers should accommodate this. Versatile light sources are essential when considering light chambers, technologies that offer both visible and UV sources can expand capabilities and, of course, the system must be ICH Q1B compliant.
Versatility and flexibility must be key features of both environmental and light chambers. Depending on the sample size, shelves may need to be adjusted to fit larger samples or greater numbers, and the provision of perforated, solid and shaker support shelves deliver the widest coverage of testing scenarios. Automation drives speed and efficiency, but also helps to protect operators. Automated shut-offs, initiated when doors are open during UV cycles, and alarms that indicate quality control issues can all help to prevent accidents and ensure safety parameters are met. In addition to automation, intuitive operational features, such as simplified programming and one-touch settings, all help to streamline workflows and minimize operator training requirements. And finally, technology design plays an important role. Features such as a small footprint and durable construction can make all the difference in busy laboratories.
Going to extremes to make drugs safe
Drug stability testing is critical for both the research and development and quality control processes needed to take a drug to market.
Creating a drug stability profile protects safety and efficacy once a drug is on the shelf, but its creation requires the use of tightly controlled parameters. By using forced degradation testing in specially designed environmental and light chambers, manufacturers can now access a fast and fully compliant way to improve their processes and deliver consistent, high-performance drugs. By selecting the right chambers and ensuring ICH compliance, drug manufacturers can take important steps to protect brand integrity and ensure patient safety.