The manufacture of a High Potent Active Pharmaceutical Ingredient (HPAPI) compound can present many challenges due to the complex handling required for toxic substances. The successful and safe manufacture of HPAPIs requires a highly skilled team with the right experience, the proper evaluation and training procedures being in place and state-of-the-art facilities. Recently, Alkermes Contract Pharma Services and a large pharmaceutical company collaborated to establish a high-volume process for Highly Potent (HP) Active Pharmaceutical Ingredients (APIs).
For the company in question, donor site capacity was an issue and the drug maker was seeking an outsourcing manufacturing partner to assure adequate commercial supply. Its product was classed as a highly potent compound and had an Occupational Exposure Limit (OEL) of 0.5 µg/m3, an Adverse Drug Event (ADE) level of 5µg/day and exhibited teratogenic and eco-toxic properties. Further, the product had a low Minimum Ignition Energy (MIE) of <3mJ and manufacturing required contained dispensing, high shear granulation with microwave drying, blending, compression and coating processes.
- Defined standard operating procedures, developing and managing a staff training program;
- Using tools to evaluate and measure exposure;
- Designing and developing containment and controls;
- Developing systems to verify effectiveness, and
- Determining and assessing the environmental impact of the active substance and associated manufacturing.
Ensuring these steps were in place was particularly important due to the fact that the Alkermes site is a multi-product facility. Concern relating to cross contamination with other products being manufactured had to be considered.
The following steps were taken to ensure containment procedures were in place to manage the safe manufacture of the HPAPI product. First, OELs were determined and a compound categorization scheme was organized. Industrial Hygiene (IH) exposure assessment was conducted, and control verification and sensitive IH analytical methods were also instituted. General and specific handling guidance, procedures and training, as well as medical surveillance regimes were instituted as well.
The Result
With these measures well understood and implemented the HPAPI product successfully tech-transferred onto the Alkermes Athlone, Ireland site. From Alkermes’ involvement with this project, a number of significant milestones were realized, including the execution of a robust containment strategy:
- Primary: high containment primary processing areas, high containment transfers and sampling areas were built (or established)
- Secondary: segregated processing rooms were built
- Tertiary: dedicated segregated suite, security access controlled, Closed Circuit Television (CCTV) remote monitoring, Heating Ventilation and Air Conditioning (HVAC) single pass air (safe change in room), double High Efficiency Particulate Air (HEPA) exhaust, pressure cascade and fogging shower were all put in place.
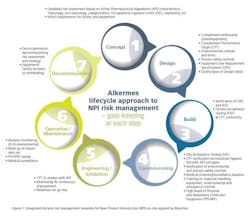
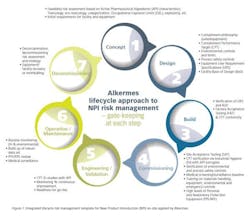
Other significant actions included organizing contained and dedicated waste water facilities, and making available on-site personal protection equipment (PPE) and respiratory protection equipment (RPE) suitable for HPAPI handling. Alkermes also installed segregated, high containment dust extraction systems and implemented an extensive training and competence development program for all staff involved in the manufacture and handling of the highly potent product. Lastly, Alkermes established a medical surveillance, proactive IH and environmental monitoring regime. Notable was the institution of a New Product Introduction (NPI) model (Figure 1) designed by Alkermes risk managers to enforce an integrated lifecycle risk management approach during all API-related operations.
Efective Approach
The use of the NPI model allowed for a robust approach to be followed when bringing HPAPIs onto the site and proved effective in implementing tech-transfers and commercial manufacture of all products including highly potent compounds for partners.
As a result of this partnership, the Alkermes Athlone site now has the capability to handle APIs to potency of 0.1 µg/ m3 at development (Discovery to Phase III) and commercial high volume scale. Alkermes now successfully handles two commercially available potent compounds on its multi-product facility in Athlone for its partners.
Published in the September 2013 edition of Pharmaceutical Manufacturing magazine