Biopharmaceutical manufacturing is one of the most complex and high-tech of all manufacturing operations. Further complexity is added due to the highly regulated and competitive nature of this market. But because the industry is so regulated, technological changes and progress in bioprocessing tend to appear evident relatively slowly, generally measured in years. Thus, many bioprocessing-related trends are not really unfamiliar. However, when external forces change, existing trends can be rapidly accelerated, as we are now seeing resulting from the COVID-19 pandemic.
[sidebar id=4]
In BioPlan’s “17th Annual Report and Survey of Biopharmaceutical Manufacturing,” we report and review the results of this extensive survey of global bioprocessing. This year’s study includes results from surveying 130 bioprocessing developers/manufacturers and 150 bioprocessing vendors. In addition, we also interviewed 26 bioprocessing company and vendor executives regarding the long-term changes and trends expected at industry responds to the pandemic. Here, we review some of the top-level ongoing trends affecting bioprocessing, including hiring and staffing difficulties, which could have long-term negative impacts on the industry.
Growth continues
Before the pandemic most major bioprocessing-related trends were related to the decades-long consistent growth of the biopharmaceutical industry, which continues at a rather steady rate of approximately 12-14 percent annual growth in revenue.
To this mix we are now seeing the impact of COVID-19 on the industry, including acceleration of many ongoing trends. This includes an upcoming wave of new facility expansions coming online for COVID-19 and other pandemic and biodefense products development and manufacturing. Already, about 1 million liters or more bioreactor capacity can be seen as being devoted to pandemic vaccines and therapeutics manufacturing. Much of this is proceeding at full scale production “at risk,” even before trials results are available. For example, Roche and Regeneron recently agreed to “set aside” a total of 140,000-liter capacity for manufacture of a candidate vaccine. Other companies are also diverting existing capacity to pandemic products. Considerable new capacity is being added, beyond the usual sizable amount continually added. For example, Samsung recently announced building a new $2 billion, >250,000-liter, “super-plant” in South Korea; and WuXi Biologics is building the largest single-use facility (48,000-liter batch; 6,000-liter perfusion) in Ireland. Although many of these projects were in-process prior to the pandemic, COVID-19 has accelerated the need.
Adapting and addressing the pandemic
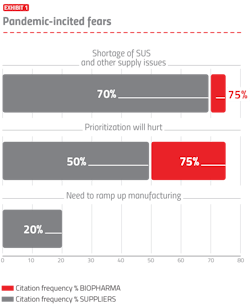
The bioprocessing sector and biopharmaceutical industry have effectively adapted to the ongoing pandemic, including continuing manufacturing and R&D, implementing needed staff and operational changes, and paying greater attention to assuring robust supply chains. Key activities, including R&D and manufacturing, are often being increased to address the pandemic. The most commonly cited fears are shown in Exhibit 1.
The top fear noted was the concern about difficulties and delays in obtaining needed single-use supplies in a timely manner. With many facilities ramping-up pandemic-related R&D and manufacturing and others purchasing more supplies to have more in storage, the preexisting shortages of many single-use system (SUS) supplies are worsening. The second most common fear cited was “prioritization will hurt,” which refers to the new fact-of-life that nearly all suppliers and many developers are now prioritizing their orders and activities, pushing pandemic/biodefense-related purchases to the front of the line. Prioritization combined with expected worsening of ongoing shortages, including high-purity polymers, will result in more facilities having longer wait time for suppliers to fill orders, particularly single-use supplies. “Need to ramp up manufacturing” relates to many facilities now rapidly building new and expanding manufacturing capacity, often at world-class scales and speculatively, even before trial results are in.
Hiring/staffing: A “perfect storm” of trends
Despite strong trends involving an increase in automation, a major confluence or “perfect storm” of complementary trends is and will result in increased problems hiring and retaining bioprocessing staff. The biopharma industry can and will manage, well enough, capacity-wise by shifting around manufacturing. New facilities and expansions can be brought online relatively quickly, generally within a few years. However, even before the pandemic, bioprocessing staff were chronically in short supply (e.g., ~40 percent of the industry is experiencing problems hiring staff in process development).
And the situation is only getting worse with COVID-19. It takes a long time to train staff, starting with undergraduate life sciences, often graduate training, and most critically, months of on-the-job training. Thus, staff expertise takes many years to develop. There will continue to be a major human capital, talent, knowledgeable staff “crunch” or shortages in the next few years.
Complementary trends that are colliding to form a worst case situation for bioprocessing hiring and staffing issues include:
- Continued rapid industry growth: Driven by consistent ~12 percent annual growth in biopharmaceutical revenue, bioprocessing activity and expenditures.
- Pandemic response: Related R&D and manufacturing is being rapidly ramped-up at dozens of facilities, with over 100 COVID-19 vaccines added to the industry’s development pipeline. Staff with virus- and vaccine-related expertise are already in short supply.
- Cellular and gene therapies coming online: These fields are seeing very rapid growth. This includes BioPlan estimating a current 500 percent shortage of bioprocessing capacity, with 5x current capacity needed (would be used if available). Hiring and staffing in these sectors is already very tight with shortages growing, with the situation made even worse by cellular therapy being particularly labor intensive.
- Biosimilars/biogenerics: The industry has recently and will continue to place emphasis on biosimilars directed to highly developed markets and biogenerics directed to lesser- and non-regulated international commerce. This includes >1,100 biosimilars/biogenerics either in development or marketed worldwide, bringing many new countries and hundreds of companies into bioprocessing. U.S./EU CMOs have generally reported seeing a 15 percent annual increase in projects in recent years attributed to biosimilars/biogenerics.
- Continuous processing: Some bioprocessing sectors are planning for adoption of continuous upstream perfusion and downstream continuous chromatography. Facilities report they are currently evaluating options or implementing continuous processing adoption. Staff with relevant experience are already in short supply.
- Experienced staff are leaving: Baby boomers, post-WWII-born staff, those with the most experience and senior positions, are reaching retirement age.
- Insufficient new staffing candidates: There are not enough undergraduate and graduate degreed individuals available and these candidates typically have no relevant experience, with it generally taking ≥6 months to train for any bioprocessing-related position. More will likely be entering these fields in response to the more positive image the biopharma industry is now seeing related to its pandemic responses.
- Growth in Asian bioprocessing: Bioprocessing activity and capacity are rapidly increasing in China, South Korea, India and other Asian countries.
Future hiring demands
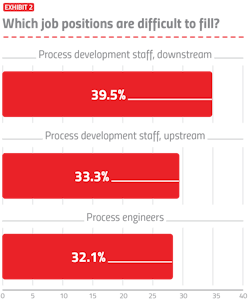
As the industry expands on many fronts, hiring will present more severe problems, especially where rapid growth is involved, such as in cell and gene therapy and pandemic vaccines. We asked respondents which job positions are currently difficult to fill (Exhibit 2). Of the 25 areas covered, downstream process development staff was again this year cited as the most difficult to fill by 39.5 percent of respondents. This was followed by upstream process development staff and process engineers.
The steady growth in bioprocessing and smooth operation of the biopharma industry could be constrained by the need for qualified staff. The needs for specifically trained staff with hands-on bioprocessing experience have remained stubbornly in place, year after year.
Shortages of experienced staff will likely worsen as experienced baby boomer staff retire, and pandemic vaccine and therapeutics bioprocessing, as well as growth in developing regions such as China, add to demand worldwide.