Instrumental in profitability
Blockbuster products have historically driven the market, and the profits of pharma companies. But since the late 1990s, many of the patents for these top-selling pharmaceuticals have expired. This has created challenges as pharma must now manufacture existing products much more efficiently to maintain profitability.
A wealth of new medications and more targeted therapies are being developed for the treatment of rare illnesses. Success has been achieved with biotechnology-based pharmaceuticals, but these products require improved monitoring and control of manufacturing processes.
New process control challenges
Today, among the world’s best-selling pharmaceuticals, more active substances are manufactured with biotechnology than with chemical processes. Because much of this production takes place in bioreactors using living cells, biotechnology processes are more complex than chemical synthesis. The stability, effectiveness and safety of the medication depend on hundreds of factors, from the air quality and the nutrients solution, to the design of the bioreactors and associated systems.
These processes require ideal conditions, especially in the bioreactors, so the cells will produce the desired active substances in sufficient amounts with high quality.
Adding to these process control challenges, pharma is one of the world’s most heavily regulated industries — and regulatory requirements have increased, especially over the past few years, and this trend is expected to continue. At the center are guidelines for assuring the quality of production processes and environments. The aim is to ensure that medications are manufactured with consistent quality.
Introducing innovative biotechnology processes while adhering to a process analysis initiative originated by the U.S. Food and Drug Association (FDA) is a requirement. And this must take place while driving towards operational excellence, forcing pharma to think outside the box while using state-of-the-art technologies and processes.
Among other things, this requires a better understanding of the manufacturing processes, and the implementation of real-time monitoring and control of quality parameters. Continuous, highly automated processes are becoming more established in new plants, in contrast to traditional batch manufacturing with manual steps, and these processes require new points of measurement delivered by improved, and often additional, instrumentation.
Starting with water
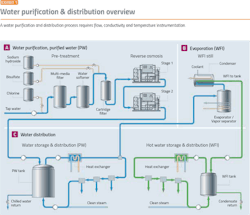
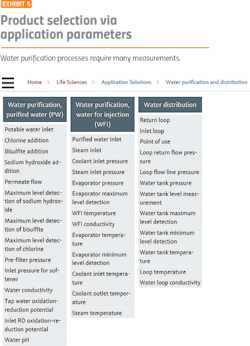
Water purification is a good place to start because it demonstrates the major issues facing the pharma industry. Before starting any pharma manufacturing process, water purification and distribution systems must be in place. Water purification processes (Exhibit 1) require:
- Flow measurement
- Conductivity monitoring
- Temperature measurement
- Convenient calibration
- Self-diagnostics and verification
Flow Measurements
To prevent bio-burden, water must be kept in motion, with the flow speed not dropping below 2 m/sec. Flow should be maintained in all portions of the water system at all times, or provision should be made for automatic flushing of water to a drain.
The actual velocity of the liquid flow is subject to discussion. The currently accepted velocity of 2 m/sec has little relationship to the prevention of microbial growth or the formation of biofilm on the pipeline walls. In piping systems that continually operate hot, any flow rate that is sufficient to keep the pipeline full and at the required minimum temperature should be acceptable.
The flow rate should also be sufficient to displace air from the high points of the piping distribution system. In systems that operate at temperatures where microbial growth can occur, a specific rate of flow will not ensure low microbial levels and other actions — such as periodic sanitization — are required to provide a suitable operation. Therefore, reliable flow measurement is fundamentally important, and these critical measurement points must be calibrated frequently to ensure accuracy.
The Endress+Hauser TrustSens RTD checks its own calibration after every sterilize-in-place operation.
Conductivity
Conductivity is one of the main quality characteristics in the distribution loop for purified water (PW) and water for injection (WFI). The limits, defined in the pharmacopeias, have to be met at all times.
Fluid conductivity is related to temperature. As the temperature of a fluid increases, the activity of the ions in solution increases. This increase in ion activity appears as an increase in the fluid conductivity. Therefore, for a fluid of a given ionic strength, the apparent conductivity will increase with an increase in temperature. For this reason, most fluid conductivity sensors need to measure and compensate for changes in temperature.
Temperature
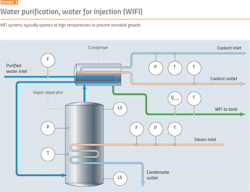
Elevated temperatures can prevent microbial growth, so it is usual practice for hot WFI systems (Exhibit 3) to operate at 80ºC. The value of 80ºC has been accepted for many years, but lower temperatures are also effective in preventing microbial growth.
Temperature should be measured at key locations within the storage and distribution system and at the storage tank vent filter. Temperature monitoring should cover normal operations and sanitization/steaming verification.
Pressure
Except during times of shut down for maintenance, critical pipeline systems should be maintained under continuous (positive) pressure, to prevent the intrusion of any contaminants. There is no minimum pressure stated in the regulatory guidelines, but the pressure should be sufficient to prevent back siphonage from any point in the system and provide adequate pressure for systems that require a minimum pressure for consistent operation. Pressure should be monitored at appropriate locations in the WFI distribution piping, such as the start and end of the distribution loop.
All instrumentation in a water purification process — and all other pharma processes, for that matter — must be calibrated and verified on a regular basis to meet FDA and international regulations.
Calibration and verification
Quality Risk Management has become a mandatory regulatory requirement for drug manufacturers. The FDA and the European Medicines Agency (EMA) publish guidelines and requirements which customers and vendors are expected to follow. Guidelines such as “Process Validation: General Principles and Practices” from the FDA and Annex 15 issued by the EMA offer input to help drug manufacturers design processes correctly.
Also, ISO9001:2008-7.6, GMP and WHO regulations and standards all require equipment and instrumentation to be calibrated or verified at specific intervals against measurement standards traceable to international or national standards.
Calibration typically requires shutting down a process every six months or so to remove and replace an instrument (Exhibit 4), then taking the instrument to a lab for calibration.
In pharma, just getting to a sensor can be a problem because of the number of surrounding devices and the need to maintain cleanliness.
An alternative way to fulfill the legal requirements is in-situ verification of the instrument. With this method, the instrument runs an on-board diagnostics program to ensure continued compliance. All relevant components of the instrument are checked to confirm and document it still meets original specifications.
Several instrument makers offer in-situ calibration and verification, and all work in a similar fashion. For example, Endress+Hauser’s Heartbeat Technology provides documented proof that a flowmeter performs according to specification. If a device is equipped with Heartbeat Technology, all test sections are monitored continuously and are part of the standard device diagnostics (sensor, front end, reference and I/O loop). If a verification is initiated, the current status of all diagnostics parameters is read and stored, with a unique identifier in the failsafe memory of the flowmeter. A verification report in PDF format is generated based on the diagnostics data of this snapshot which can be downloaded, printed or stored externally for audit documentation.
Recent developments
Developments in temperature sensor technology now make it possible for an instrument’s sensor to determine if it needs calibration, thus eliminating unnecessary lab calibrations.
For example, many reactors, tanks and vessels are cleaned and sterilized between each batch with Sterilize in Place (SIP) procedures. SIP is a sterilization process that consists of injecting steam into the vessel and holding temperature around 121°C for up to an hour. An RTD temperature sensor can take advantage of this constant temperature during SIP to check its calibration.
This is accomplished using a physical fixed point known as the Curie Point or Curie Temperature. The Curie Point is the temperature at which the ferromagnetic properties of a material abruptly change. The sensor contains an embedded reference with a Curie Point of 118°C. As the temperature cools from 121°C to below 118°C, the RTD will automatically perform an in-situ calibration.
When the Curie temperature of 118°C is reached, the reference sensor transmits an electrical signal. At the same time, a measurement is made in parallel via the RTD’s temperature sensor. Comparison between these two values effectively is a calibration identifying any errors in the temperature sensor. If the measured deviation is outside set limits, the device issues an alarm or error message that is also indicated via a local LED on the sensor. The calibration data acquired is sent electronically and can be read using asset management software. This also enables an auditable certificate of calibration to be created automatically.
Being able to minimize unnecessary calibrations and document data for audits can save a company time and labor.
As highly automated processes become more and more prevalent in the pharmaceutical industry, and these processes require instrumentation, calibration and verification to meet FDA and international regulations. Fortunately, modern instrumentation and supporting software from major instrument companies are easing the burden.