If you’re a Game of Thrones fan, for many years you’ve heard that “winter is coming.” When it comes to biosimilars, it seems that we are finally facing it. More or less a decade ago, biosimilars were the incoming future, and now, across the globe, we can decisively say, that future is today.
A great growth is expected in the biosimilar market due to biologic blockbuster patent expirations, opening new markets and opportunities potentially worth billions of dollars. Moreover, biosimilars have big expectations on them — mainly from patients and governments — because they promise to reduce prices up to 50 percent when compared to the original biologic medicines.
That’s why biosimilars are getting stronger every day and are expanding exponentially year after year all over the world. Specifically, I’d like to focus on the Latin America market.Why LATAM?
For years the news has been about big foreign multinational corporations trying to penetrate the growing LATAM pharma market. Companies were drawn in by the promise of expanding local economies during the first decade of this century, skilled and cheap labor, the boom in generics, a big government coverage in medicines, and weak patent control (nowadays more sophisticated and regularized). But the strong local pharmaceutical industry has faced large multinationals for many years, and in many cases local companies formed regional groups of companies. The local companies, which historically served only their home-country markets, have consolidated and expanded into other Latin American countries.
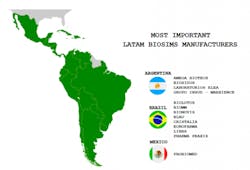
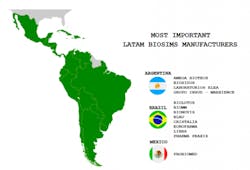
It is thus not surprising that there is a flourishing biosimilar industry in Latin America, with its own research, development, production and commercialization capacity. The three most prominent biosimilar manufacturing countries in Latin America include Argentina, Brazil, and Mexico. Due to its population and market magnitude, Brazil is spearheading the biosim process.
Challenges remain
But like biosimilars on the global level, Latin American biosimilars are up against numerous challenges. On the one hand, there are the high costs (R&D and clinical trials) of staying at the forefront of biotechnology, taking into account that, economically speaking, it’s a region of developing countries with short-term economies and policies.
In the near future, the main efforts should be put on developing the necessary infrastructure to evaluate the analytical and clinical information required for the right registration, comparative quality studies, the safety and efficacy of the biosimilar, as well as the balance between risks and benefits versus the “original” biotech product.
Technical issues like pharmacovigilance, biosimilar effectiveness, and safety that have to be carefully considered and handled if companies want to continue riding the crest of the biosim wave.
Countries in the region should increase their efforts in pharmacovigilance, train more regulatory personnel, and generate reports to improve systems for capturing and analyzing data. If we take into account that biosimilars have been newly commercialized and thus historic data is scarce, it is important that biosimilars have adequate regulation and standardization.
At the beginning of 2000, the process for registering biosimilars was quite simple, and therefore a high number of them were approved. Nowadays the story is different, and worldwide speaking, the referents on biosimilar guides and regulatory issues are the FDA (USA), EMA (European Union), and WHO (World Health Organization). That’s why the agencies of Argentina (ANMAT), Brazil (ANVISA), and Mexico (COFEPRIS) used the FDA, EMA, and WHO guidelines as references to launch their own regulations.
Although some Latam countries are ahead on the trend, there are still many other countries in Latin America that are still regulating the biosimilar market and creating their own guides.
Given that biological products consume a substantial proportion of health care national budgets , the financial pressure to adopt biosimilars is high and should not be taken lightly. For example, in Canada, biosimilars are called Subsequent Entry Biologics (SEBs), and are subject to the same regulation as the original biological drugs, they are also classified as new drugs and they are not considered identical to the original.
If we extrapolate it to Latin America, it is important that socio-economic pressures don’t influence regulatory processes and count with high standards if we have in mind that they are new drugs for which there is no history of risk, benefit, security, and effectiveness. And that’s why it’s important to have well-designed guidelines.
Nowadays biological drugs are responsible for approximately 50 percent of the hospital bills worldwide, so no one doubts the benefit that biosimilars could contribute to health systems. This benefit is even greater in less developed countries, where the use of biosimilars allows many patients to have access to complex treatments.
Finally we can point that the expansion of biosimilar companies in Latin America benefits this market even more, since it adds more players to the game, bringing more competition which will lead to better prices, new drugs, and more alternatives at a global level.