Excipients play a key role in helping pharmaceutical manufacturers serve patients better through improved compliance and efficacy of treatment. They also help reduce developmental costs and provide opportunities to differentiate products through new modes of drug delivery.
Experts identify several trends driving excipient demand: solubility and bioavailability challenges, as well as the desire to increase the lifecycle of a drug, improve manufacturing efficiency and address the growing bioequivalence market.
• Provide access to new treatments and improve the efficacy of new chemical entities (NCEs), which are typically less soluble;
• Address specific needs for pediatrics and geriatrics – taste masking, ease to swallow, alternative dosage forms (orally disintegrating tablets);
• Improve compliance – controlled release to reduce frequency of administration and extend duration;
• Provide different product options – alternative routes of delivery/dosage forms;
• Improve ease-of-use – no need to take with food / water, no need for refrigeration, lower risk of overdose due to consumption of alcoholic beverages; and
• Improve production technologies and realize more efficient and effective processing.
SOLUBILITY AND TARGETED DELIVERY
The solubility and permeability of many new chemical entities - which are very often highly potent - is a key issue for the development of new drug formulations. As a result, the industry is in urgent need of new approaches to drug development and tailored drug delivery. The 2017 Nice Insight Pharmaceutical Excipients Survey indicates that solubilizers - commonly used to improve the solubilization of hydrophobic substances and to increase bioavailability - experienced a 49 percent increased use over the last year among survey respondents.
Riermeier says solid dispersions and solid solutions, combined with targeted drug delivery, are the future for oral drug delivery. “If you have a BCS class IV API with poor solubility and poor permeation, increasing the solubility alone may not solve all your problems, but delivering the now soluble drug to the right area of the GI tract may be able to boost bioavailability. Hence, methods for absorption window mapping gain importance in formulation development,” he says.
As an example, Riermeier says that Evonik’s functional polymer excipients, EUDRAGIT, can significantly increase bioavailability through delivering the active to the appropriate area of the GI tract where absorption is the highest or to the site of action where the active is needed.
Chattopadhyay says that leveraging the characteristics of excipients to modify the release profile of a drug can increase the lifecycle of that drug.
“In parenteral drug delivery, there is a greater focus on developing more advanced complex formulations such as liposomes and polymer-based microparticles, allowing for specific drug targeting or extended release, where a single injection can provide the dose for a week, a month or even longer,” says Riermeier.
BIOSIMILARS REQUIRE NEW EXCIPIENTS
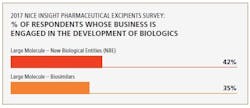
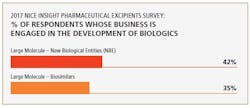
Biosimilars are driving demand for excipients used to manufacture biologicals. In fact, the 2017 Nice Insight Pharmaceutical Excipients Survey also indicates that large-molecule (biosimilars) manufacturers represent a growing group of excipient purchasers. “Thus, excipients that can mitigate biological production risks and improve process yield are in high demand,” says Chattopadhyay.
The number of biological actives coming off patent will increase in the near future. Developing biosimilars is significantly more challenging than conventional small molecules as different physico-chemical properties and specific sensitivities to regular manufacturing parameters have to be handled, explains Riermeier. “Biotechnology-based products require greater attention to stability in the dosage form as well as unique approaches to overcome biological barriers to target site delivery,” he says. “New types of excipients and innovative formulation and manufacturing technologies offer new opportunities to formulate these drugs with high bioavailability.”
MULTI-FUNCTIONAL EXCIPIENTS MAKE BETTER PILLS
As the number of difficult-to-develop compounds continues to rise, the industry is taking bolder steps in evaluating non-conventional technologies for effective delivery to mitigate risks while enhancing the efficacy of drugs and bringing these compounds to the market much faster.
“Today, more than ever, pharmaceutical formulators are seeking ways to improve the manufacturing process and product quality through the use of multi-functional excipients,” says Chattopadhyay. “Multi-functional excipients play an important role in innovating delivery technologies and helping in-line extensions of marketed drugs.”
Multi-functional excipients can help pharma manufacturing by improved flowability, enhanced compressibility, improved bioavailability, particle size distribution and reduced dust generation, etc. Aware of their benefits, the use of excipient combinations has grown 56 percent among respondents to the 2017 Nice Insight Pharmaceutical Excipients Survey.
Additionally, Riermeier says that multi-functional excipients represent significantly less development costs and regulatory hurdles than creating completely new excipients. “Multi-functional excipients can accelerate or even enable the development of new products that otherwise would not be possible,” he says.
For example Evonik’s newly launched EUDRAGIT FS 100 is a solid version of existing EUDRAGIT FS 30 D. This new multi-functional version allows pharmaceutical companies to use the polymer in many new applications, such as hot melt extrusion, solvent spray-drying and solvent coating, which Riermeier says was impossible to achieve in prior decades where only the aqueous version was available. “This kind of multi-functionality allows overall new development possibilities within the pharma industry,” he says.
THE COST OF USING EXCIPIENTS
Whether it’s improving solubility, enhancing biosimilar delivery or using multi-functional excipients, pharma manufacturers always want to find ways to reduce costs while improving manufacturing efficiency. In fact, the 2017 Nice Insight Pharmaceutical Excipients Survey shows that affordability is among the top-five purchasing criteria when choosing an excipient supplier.
Excipients can be a key tool to help address the desire for low-cost medicines as they can significantly improve production efficiency. For example, BASF’s Kollicoat IR has a polymer structure with low viscosity and high film flexibility that allows the coating to be applied much quicker, resulting in a homogenous flawless surface, explains Chattopadhyay.
“Excipients are usually not the primary cost driver for drug products, but they can play a vital role with respect to the overall therapeutic costs and efficacy,” says Riermeier. “A versatile excipient combined with the know-how of well experienced drug formulators can reduce the development costs of new drug products significantly and increase the chance to hit the right therapeutic window.”
Risk goes hand-in-hand with cost. As the pharmaceutical industry struggles with high failure rates and enormous development costs, companies want to keep the risk as low as possible. One way to do this is to use existing excipients. “The majority of pharmaceutical companies prefer using existing excipients with extensively documented use in previously marketed products rather than new excipients that may require cost-prohibitive toxicology studies,” says Riermeier. “This is why co-processed excipients and new technology platforms using existing excipients are gaining market share.”