Lean in the Lab: Build it Right, and It Will Come
While basic Lean concepts are simple and can be extremely effective, many companies – in pharmaceuticals and other industries – have struggled to gain the same kind of benefits in a laboratory environment as achieved in manufacturing. Pfizer has taken the traditional concept of Lean manufacturing and, with some innovation and collaboration, reinvented it as a replicable process easily adapted across Quality Control (QC) laboratories in its global supply network of more than 70 sites.
Pfizer has been applying Lean techniques in QC laboratories for a number of years, yet the concept created some early skeptics. How could a Lean approach apply uniformly across such a complex and highly regulated business? At each site, there could be hundreds of analytical methods, many configurations serving different countries and high product volume variability. The end result product could take various dosing forms, including tablets, caplets, capsules, liquid-gels, ointments, creams, gels, liquids and injectables. Was it even possible to apply Lean tools, such as leveling of incoming workload and standardization of work tasks, to gain the desired improvements in productivity, speed and quality?
Building the House of Lean Laboratory
What won over the skeptics and allowed the process to work throughout the Pfizer Global Supply organization was applying traditional Lean concepts in an untraditional way. The creation of the House of Lean Laboratory was established as a business objective in 2009; in 2010 it became a reality.
Like Lean itself, the basic concept was simple:
1) Begin with input from a broad cross-section of functions and locations
2) Create a standard approach with enough flexibility to be adapted to the specific needs of individual sites and lab types
3) Make it easy for each site to implement Lean initiatives by packaging the elements in an accessible way and offering a one-stop shop for information and applications
4) Address cultural and behavioral aspects, which can be a make-or-break success factor
5) Include components to ensure sustainability and foster continuous improvement
Designed with the Toyota House of Quality in mind, the Pfizer House of Lean Laboratory began taking shape in January 2010. A workshop, held in Puerto Rico, was attended by Pfizer representatives from 19 sites, spanning all seven business units (primary care/oncology, established products, specialty/biotechnology, emerging markets, consumer healthcare, nutrition and animal health), along with relevant center functions.
This was not simply a sharing of best practices. The work at hand was to develop a process at the corporate level that could be rolled out across all products, all divisions, and all locations. The approach would be open and flexible, with the benefits to adoption being so clear as to create an internal “pull” from the sites.
The goals of Lean Labs were established as follows:
- Improve productivity within the QC laboratory, with reduced costs of operations, reduced lead times in delivering results to the customer and greater consistency of result delivery to the customer
- Improve first-time quality, thereby reducing laboratory investigations and rework
- Build a culture of continuous improvement to sustain business benefits from the customer down to the analyst level, drawing on new and innovative ideas, and sustaining its impact with mindset and behavior transformations across the laboratory
- Develop a standard but flexible, holistic Lean Laboratory design that could be easily replicated and rapidly deployed across the entire global supply network
Lean Labs was to be something everyone would want to undertake because it would make their work more efficient, more effective and more profitable.
Three Tiers
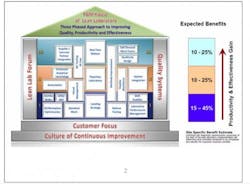
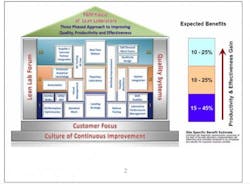
The structure of Lean Labs took the form of a typical house. (See Fig. 1.) It sits on a solid foundation of customer focus (both internal and external), where designs are based on customer expectations for speed and quality, and a continuous-improvement culture that promotes long-term sustainability of the design.
Lean Labs is supported by pillars of Quality Systems that align with the improvements undertaken and a Lean Lab Forum for site-to-site support and sharing of information and best practices.
The three tiers correspond to the levels of a house, with each level containing a number of concepts, or “rooms.” Each room is set up the same way so people know what to expect and how to navigate to the information they need. Rooms contain an overview that defines and sets the scope for implementation, noting resource requirements and constraints. There is step-by-step implementation guidance, site-specific examples as models to show what others have done, and links to tools and templates.
Level I begins with the basics: simpler approaches requiring less investment that can be implemented right away. The expected benefits at this level are a 15% to 45% improvement in productivity and effectiveness.
These benefits come from:
- The leveling of incoming workload and standardization of work tasks
- Optimization of the workplace in labs with 5S and organization strategies, work cells and visual management tools
- Lab performance management through metrics, Key Performance Indicators, “gemba” walks through laboratories to identify best practices, and shift huddles
- Method improvements to reduce repeated and unnecessary testing
- A flexible cross-trained workforce
- Error reduction through the use of human performance models
Level II may require longer implementation timelines because these activities may require capital investment or regulatory filings. They can contribute 10% to 25% productivity and efficiency gains, largely through:
- The adoption of new analytical technologies and integrated laboratory automation for paperless processes, rapid information transactions, access and decision making.
- Additional strategies include method improvements that focus on consolidation and harmonization of test methods, use of electronic visual management systems and initiation of quality control at line testing, wherever possible.
Level III efforts can result in 10% to 25% in gains, although these tend to be longer term and more strategic in nature. These techniques include:
- Real Time Release methods implemented with Quality by Design (QbD) and Quality Risk Management (QRM) to reduce or eliminate lab testing
- Additional method improvements managed by QbD and QRM to make step changes
- Quality control testing at the line when full Real Time Release is not possible
- System integration with suppliers and external partners to facilitate data sharing and information flow to reduce end-to-end lead times
- Self-directed work teams managing all aspects of daily lab operations
Each site begins its Lean journey by taking a standardized online self-assessment. Results and rankings are automatically tabulated and color-coded to show priority areas that need attention. There’s no guessing about where to start when specific priorities are clearly identified and highlighted. Each site proceeds at its own pace, tailoring its approach according to need, while drawing on the standard tools and templates that are accessible to all through a SharePoint collaboration portal.
Making access easy was paramount, and any Pfizer site can quickly link to step-by-step implementation processes, tools, templates and examples through a single document. The toolbox also includes assessment processes that allow sites to track the current level of completion for targeted Lean Laboratory concepts.
Interwoven throughout all the rooms and levels and pillars is the concept of culture: the necessary mindsets and behaviors that make Lean sustainable. Culture encompasses the way people think, feel and conduct themselves in the workplace, both individually and collectively. The initial site transformation diagnostic assesses the existing mindsets and behaviors – through surveys, focus groups, interviews – and with the recommended tools and activities, problem spots can be addressed so they don’t become barriers to success.
Dramatic Change in Short Order
The implementation of Lean Lab concepts quickly results in dramatic change:
- From limited flexibility of resources in labs to the development of multi-skilled analysts who can wear several hats
- From misaligned metrics that can drive inefficient behaviors to customer-focused team performance
- From excessive levels of waste in materials, time and resources to less waste, fewer non-value-added tasks and improvement in first-time quality
The initial goal in introducing Lean Labs in 2010 was to advance the concept globally across Pfizer Global Supply and begin formal Lean Lab Level 1 in 12 sites during the year. A total of 14 sites actually initiated Level 1 that first year. Significant savings were achieved in this first phase of implementation across all Pfizer Global Supply sites.
Sustaining, Accelerating, Replicating
With implementation at the first 14 sites rolling, the focus broadened in 2011 to begin formal Level 1 concept training at more sites. An implementation strategy was developed using onsite and remote coaches and contractors to allow sites to drive activities at their own pace based on their specific priorities, as identified in the standardized self-assessment. Additionally, an accelerated support program was launched to focus on targeted, high-benefit opportunities at a number of specific sites.
Design reviews are used to track progress and understand if additional opportunities remain, and formal success checks are completed at advanced sites to ensure the work being done is sustainable and to identify any new best practices to include in the collaboration materials, which are continuously improved.
To leverage the full value of Lean Labs, replication across sites is key. Success factors involve creating buy-in and “pull” from each site. The concept for answering the “What’s in it for me?” question is the creation of a “burning platform.” Basically, this means laying out how Lean Labs can help each site meet its aspirational performance goals and metrics.
Every team at every site needs to be engaged in the process and focused on the benefits to be gained. Then they need ready access to Lean Lab tools and materials to make it as easy as possible to work through what needs to be done. These tools were created with both standardization and flexibility in mind, allowing for customization at individual sites depending on need. One size could never fit all, so allowing each lab to tailor its approach was essential.
Perhaps the most important replication success factor has been management support. Introducing Lean Labs as a corporate objective highlighted its importance and aligned all sites toward this goal. Such visible support encouraged the sharing of resources across the organization, wherever and whenever needed, including the development of tools and adaption for sites where English is not the primary language.
Traditionally Lean in Untraditional Ways
A generic Lean approach would never be able to deliver the desired benefits in a QC laboratory setting. While most of the key principles apply, there are unique challenges involved and careful adjustment is required.
To raise the productivity and effectiveness of labs with Lean-based concepts takes a true transformation. It takes an understanding of why a Lean approach is needed and how it can create a competitive advantage within an organization. Vision is required to create a standardized, yet flexible, holistic approach and to lay out a roadmap of how and when to implement the program. Finally, there needs to be a process for sustaining and replicating success – and that requires winning the hearts and minds of the people involved. With their engagement and ideas, Lean Laboratories can lead to extraordinary, positive and sustainable change within the organization.