Orally inhaled and nasal drug products (OINDPs) are among the most common manufactured pharmaceuticals. Some examples of OINDPs are nasal sprays, metered dose inhalers, dry powder inhalers and inhalation solutions. But foreign particles can end up in these products during the manufacturing process, potentially affecting their delivery, safety or efficacy. Foreign particulate matter (FPM) can come from active ingredients, excipients (inactive ingredients), container or closure components, or from the formulation, environment or manufacturing process of the drug itself; they also can come from the process of actuating the drug product delivery device. Enumerating these particles is an enormously important aspect of product production.
The United States Food and Drug Administration (FDA) has strictly regulated the manufacturing of injected liquids products, setting important standards for the analysis and control of unwanted particles. USP 788 is a standard developed by United States Pharmacopeia; it details the level of acceptable FPM in injection liquids, setting out two methods of analyzing particulates. Many OINDP manufacturers have adopted these standards or similar ones in their own manufacturing processes to ensure the highest levels of product purity. One of the two accepted methods described in USP 788 is microscopic particle counting. It is among the most powerful and commonly used tools for identifying, counting, measuring and analyzing micron-range foreign particles in pharmaceutical product manufacturing.
USP 788 lays out careful instructions for testing medication units, setting limits for how many particles of various sizes (measured in microns) are permissible in each unit. It also provides guidance as to what kind of microscope should be used in this type of examination and analysis. Particle counting may be accomplished using manual, semi-automated or fully-automated microscope systems, but the microscope must meet the following standards:
- The microscope must produce 100x (+/- 10x) total magnification
- Two illuminators are required. One is an episcopic (reflected light) brightfield illuminator internal to the microscope; the other is an external, focusable auxiliary illuminator adjustable to give reflected oblique illumination at an angle of 10° to 20°
- The microscope must have a mechanical stage capable of holding and traversing the entire filtration area of the membrane filter (i.e. 25mm or 47mm)
- The system must be calibrated with a stage micrometer that was certified by a domestic or international institution.
Manual Microscope-Based Particle Counting
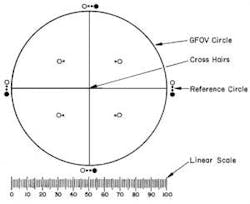
The manual method of counting particulate matter has been used for decades. According to the government guidelines, a manual microscope particle counting system must include a circular diameter reticle (also known as a graticule) designed specifically for this purpose and calibrated using a certified stage micrometer. The reticle is symmetrically divided into four quadrants by crosshairs; each quadrant shows transparent and black reference circles 10µm and 25µm in diameter at 100x magnification. The reticle also must contain a linear scale graduated in 10µm increments (Figure 1).
The operator scans each entire filter or other standard-size substrate using the reticle, carefully comparing any particles seen under the microscope to the reference circles and keeping track of the number of particles of various specific sizes (more than 10µm and 25µm in diameter, respectively, for instance). Operators are then required to enter results into workbooks, create reports and file pertinent data into either electronic or paper folders.
Needless to say, detecting, measuring and keeping an accurate count of particles of various sizes in this manner can be tedious, time consuming, subjective and prone to human error. For this reason, many pharmaceutical companies have moved to semi-automated or fully automated systems.
Semi-Automated Systems
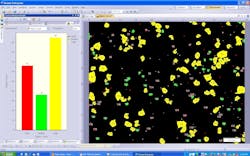
With of the addition of modern image-analysis software and high-resolution microscope digital cameras, semi-automated particle counting is now available for almost any microscope that has a standard camera mount (Figure 2). In a typical semi-automated configuration, images of foreign particles are acquired at various fields of view over the entire area of the filter. Image analysis software is then used to analyze each individual image. First, each image is segmented by the image-analysis software into particles vs. non-particles, based on user-definable gray-scale threshold values.
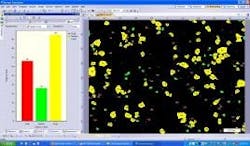
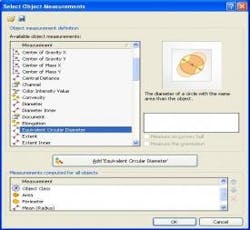
Next, using advanced image processing algorithms, morphological filters are applied to each image to automatically separate touching particles that would otherwise falsely be counted as one (Figure 3). Last, the operator selects the size parameter (area, diameter, equivalent circle diameter, feret, perimeter, etc.) or morphological parameter (aspect ratio, convexity, elongation, shape factor, circularity, etc.) of interest to be calculated on each particle (Figure 4). The user has the ability to set a size or morphological range (i.e. exclude any particles <10µm) and also can classify particle results into bins (i.e. Class 1: ≥ 10µm; Class 2: ≥ 25µm).
Semi-automatic particle detection offers numerous advantages over counting and measuring particles manually. Perhaps the most significant benefit is the increased accuracy and repeatability gained by minimizing the variation that can creep in through manual examination of each filter.
Further, images and resultant data can be archived automatically into an integrated database. Operators can query this database at any time for both raw data and trends information that may affect quality assurance procedures or process control. Users or managers can generate electronic reports with the click of a mouse, automatically inserting particle images, spreadsheets and histograms.
Fully Automated Microscope-Based Particle Counting
While it may take as long as an hour to perform a particle count on a 47mm filter with a manual microscope-based particle counting system, semi-automated image-analysis techniques can do it in a fraction of the time.
Even more impressive are fully automated microscope-based particle counting systems, which can complete such a task in as little as three minutes (Figure 5). New developments in hardware and software have brought such automated systems to the attention of pharmaceutical manufacturers, and they are now in place in many production facilities. While semi-automated counting relies on the operator manually positioning the XY stage underneath the microscope and then manually triggering image capture at various fields of view, the new systems automate the entire workflow, resulting in enormous savings in time and great gains in repeatability and precision.
With a motorized XY scanning stage and specialized image-analysis software, a fully automated microscope-based particle counting system scans the entire area of the media to be examined (usually the area of a 25mm or 47mm filter), with the image-analysis software triggering image acquisition automatically at each frame. Since the image-analysis software uses application-specific algorithms to determine how many images are to be acquired over the area of the media (as well as the spatial XY position of each frame), the operator no longer needs to figure out how many, or from where, images are to be acquired.
Should a particle lie on the border between image frames, the software automatically reconstructs the particle among the image frames by carefully “stitching” it together from various segments, without inaccurately truncating the particle and/or counting as a duplicate. In a fully automated microscope-based particle counting system, focus is achieved at each frame via a high-performance, advanced laser-based autofocus module, which can perform accurate autofocus on high contrast samples within milliseconds.
As a cost-effective alternative to the highly sophisticated laser system, some fully automated image analysis software offers “Predictive Focus” algorithms, which are quite successful at compensating for any potential linear or non-linear variances in sample topography (Figure 6). Predictive focus works based on a series of in-focus points that the operator defines prior to the scan. Once that step is completed, the image-analysis software is able to calculate an extrapolated Z-axis position at every frame, which is then communicated back to the microscope, allowing the system to accurately predict points of focus across the filter media.
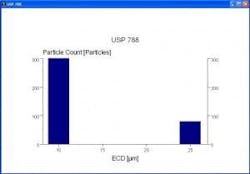
Fully automated systems offer additional benefits even beyond these performance gains. All relevant data including spreadsheets, histograms and reports can be generated in compliance with any company-defined or other predetermined standard (such as USP 788) (Figure 7). Data can be archived into an integrated database automatically, virtually eliminating the potential for data loss that can occur when operators forget to input data.
Administrators can customize and define database fields according to their specific experiment requirements and workflow. Later, users can search by any of these fields (such as experiment number, operator name, customer name, date range, etc.) to find important information or to look for patterns. In addition to such data management tools, some of the more advanced automated microscope-based particle-counting systems also are designed to comply with 21 CFR Part 11 guidelines concerning electronic records and electronic signatures.
Though their capabilities are immense, operating the newest fully automated systems is quite simple. In a typical pharmaceutical manufacturing environment, an administrator or more advanced user sets up the system, pre-defining such experimental criteria as segmentation thresholds, scan areas, report templates and database settings. Non-qualified users can be locked out from adjusting these critical settings.
Users find that their interface is streamlined, intuitive and extremely user friendly. They simply put the filter media in a fixture under the microscope, enter a few key data points with the touch of a button, and press “Start.” In just moments, the automatic particle detection is complete, all the resultant data and reports are automatically generated, and all the data has been archived into the database.
Optical microscopes are commonly used for particle counting to quantify the level of foreign particulate matter in orally inhaled and nasal drug products and injection liquids. With the addition of a modular, high-resolution digital camera, along with modern image-analysis software and a motorized microscope XY scanning stage, this process can be semi-automated or even fully automated, significantly increasing throughput, accuracy and repeatability.
Further, relevant data and reports can be generated automatically and archived electronically.
Data is easy to analyze for enhanced decision-making during the production process, and is produced in compliance with either company-defined or 21 CFR Part 11 standards. With these developments, pharmaceutical companies are increasingly looking at fully automated systems to help streamline the analysis, production and verification of manufacturing procedures and standards for these types of pharmaceutical products.
About the Author
Mario Gislao is Applications Specialist, Scientific Equipment Group, Industrial Microscopes, for Olympus America Inc. He can be contacted at [email protected].
References
1. Thomas A. Barber. Control of Particulate Matter Contamination in Healthcare Manufacturing. Interpharm Press, 2000, pp 115-129. http://books.google.com/books?id=1F1Rs_KnH3AC&pg=PA116&lpg=PA116&dq=circular+diameter+graticule&source=bl&ots=vzsF_v2RxV&sig=reqKsqp_cz0yXTI0xsXoAZ8mCJs&hl=en&ei=3kbQS_HTLoH58Aa2w8maDw&sa=X&oi=book_result&ct=result&resnum=2&ved=0CA8Q6AEwAQ#v=onepage&q=circular%20diameter%20graticule&f=false
2. James Blanchard, et al. “Foreign Particles Testing in Orally Inhaled and Nasal Drug Products,” Pharmaceutical Research, Vol. 21, No. 12, December 2004 (© 2004)
3. United States Pharmacopeial Convention. <788> Particulate Matter in Injections http://www.usp.org/pdf/EN/USPNF/788ParticulateMatter.pdf.
4. Lee M. Nagao, et al. “Aspects of Particle Science and Regulation in Pharmaceutical Inhalation Drug Products,” Crystal Growth & Design, 2005, 5 (6), pp 2261–2267.