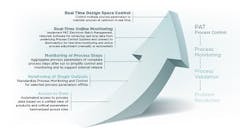
Validation
Validation involves the collection and evaluation of data — from the process design stage through commercial production — which establishes scientific evidence (proof) that a process is capable of consistently maintaining the necessary level of compliance. Here you will find content that discusses the qualification of systems and equipment.