HP: Serializing the Tablet, Courting Big Pharma
By Paul Thomas, Managing Editor
Pfizer recently announced that it had begun a web-based service by which distributors, wholesalers and retailers could log on to the web and verify a drug bottles EPC code. (See "Pfizers Staver on RFID: 'Technology Isnt the Only Solution' ") Hewlett-Packard is behind an effort to offer a similar service, but one that would allow consumers, if they so desired, to use the Internet to verify each and every pill they take. The initiative would use software and services provided by Verify Brand, Inc. (Minneapolis), which is partnering with HP and several other vendor companies to offer full-service tablet- or capsule-level anticounterfeiting solutions.HP has been working on its inks and fluids for on-tablet printing for some time now, particularly at its Puerto Rico facilities. It is in talks with Pfizer and other major pharmaceutical manufacturers to apply the technology to a product and have it approved by FDA, says Homayoun Akhbari, strategic alliance manager for Hewlett-Packard.We cant just make one pharmaceutical ink and have it work with any pharmaceutical product, says Akhbari. It has to be compatible with different substrates of tablets.The equipment to serialize the tablet with non-contact, inkjet technology has already been developed in collaboration with inc.jet (Norwich, Conn.), which is providing the printing cartridges and components, equipment maker Efficient Automated Machine Corp. (Long Island City, N.Y.) and system integrator Luciano Packaging Technologies (Somerville, N.J.).
Pfizer recently announced that it had begun a web-based service by which distributors, wholesalers and retailers could log on to the web and verify a drug bottles EPC code. (See "Pfizers Staver on RFID: 'Technology Isnt the Only Solution' ") Hewlett-Packard is behind an effort to offer a similar service, but one that would allow consumers, if they so desired, to use the Internet to verify each and every pill they take. The initiative would use software and services provided by Verify Brand, Inc. (Minneapolis), which is partnering with HP and several other vendor companies to offer full-service tablet- or capsule-level anticounterfeiting solutions.HP has been working on its inks and fluids for on-tablet printing for some time now, particularly at its Puerto Rico facilities. It is in talks with Pfizer and other major pharmaceutical manufacturers to apply the technology to a product and have it approved by FDA, says Homayoun Akhbari, strategic alliance manager for Hewlett-Packard.We cant just make one pharmaceutical ink and have it work with any pharmaceutical product, says Akhbari. It has to be compatible with different substrates of tablets.The equipment to serialize the tablet with non-contact, inkjet technology has already been developed in collaboration with inc.jet (Norwich, Conn.), which is providing the printing cartridges and components, equipment maker Efficient Automated Machine Corp. (Long Island City, N.Y.) and system integrator Luciano Packaging Technologies (Somerville, N.J.).
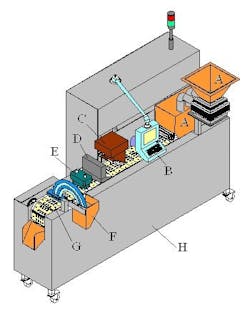
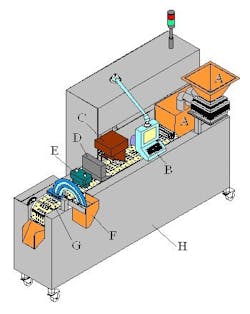
B. System control screen
C. Ink cartridge printing station
D. NIR drying station
E. 100% vision camera inspection system
F. Tablet and capsule individual reject station with verification
G. Tablet and capsule firm grip conveyor puck system design
H. System fabricated in all stainless steel, pharmaceutical-grade materials