Recently, a major pharmaceutical manufacturer called me to help resolve a major tablet compression capacity issue. Product demand was increasing but capacity lagged behind, even after new equipment was purchased to speed up the compression machine. Operator performance was inconsistent, and increasing the campaign sizes only led to larger inventories, presenting entirely new, and unwelcome, challenges. Lothad been delayed, deadlines missed, and many heated phone calls ensued.
A clear solution for this fairly common scenario was to apply Overall Equipment Effectiveness, or OEE, one of the most effective tools that pharmaceutical manufacturers can use to manage and improve the performance of key capital assets. OEE is fairly widely used in areas such as pharmaceutical packaging, but is also being applied to processing steps. This article examines how OEE and SMED were used to manage a tablet compression machine, resulting in a 50% reduction in changeover times and a 75% decrease in changeover variability.
Unfortunately, we cannot divulge the name of the manufacturer involved, but offer the case study as an aid to companies looking to use OEE to improve unit operations and pharmaceutical manufacturing performance.
OEE, which evaluates equipment performance, availability, and product quality, systematically identifies opportunities for improvement. In this case, changeover times rather than line performance was identified as the largest cause for overall performance and variability in production. Elements of Lean and Six Sigma methodologies such as Define, Measure, Analyze, Improve and Control (DMAIC) and Single Minute Exchange of Dies (SMED) were then used to methodically eliminate waste from the changeover process without sacrificing quality or endangering compliance. In addition, standardized work, direct observation of the line, root cause analysis, and statistical process control (SPC) were used minimize variability and establish a new changeover baseline.
The Road to OEE
One of the first actions was to accurately measure the “current state”. As with any major piece of capital equipment, an equipment utilization measurement is needed. The best metric for this is OEE because it provides specific information on where to attack waste.
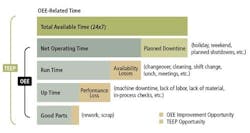
Relative to common measurements such as uptime, units produced, and production speed, OEE helps us understand what could have been produced and where the sources of inefficiency are located. OEE is composed of three main categories: Availability, Performance and Quality.
The Availability is the time the equipment is actually run versus the time it could have been running. A low availability rate reflects downtime from: changeovers, cleaning, shift changes lunch, breaks, etc.
The Performance is the quantity produced versus the potential quantity produced at a given run rate. A low performance rate reflects speed changes from: a slower line speed, machine downtime, lack of labor etc.
The Quality is the amount of good product versus the total product produced. A low quality rate reflects defects from: scrap and rework
In Figure 1, we are able to see how Availability, Performance and Quality are related in determining the overall effectiveness of a piece of equipment. As the bottom line output, “Good parts,” shows, the overall output of the equipment is only a fraction of its potential output. Availability, Performance, Quality can be addressed individually or in conjunction in order to improve overall productivity. (Note: TEEP, Total Effective Equipment Productivity, equates to OEE when scheduled 24/7. TEEP is not discussed here.)
In the pharmaceutical industry, many pieces of equipment have long set-up times because equipment must be torn down, cleaned up, and set back up to cGMP criteria. This time loss can represent a major loss of productivity and asset utilization (the Availability portion of OEE).
In this case, we helped identify and remove changeovers as a major contributor to Availability loss for the compression machine.
Define Stage: “Why Can’t We Make More?”
Faced with uneven output, we utilized the Six Sigma DMAIC problem-solving methodology and Lean tools to help the client identify and tackle the variable performance of the tablet compression machine. In order to best understand the usage of the equipment, historical OEE measurements were cobbled together from batch records and other available data. From this analysis it was clear that Availability losses and, more specifically, changeover times (and not throughput) was the key factor limiting the performance of the machine. Analysis of these records indicated that the shift average for changeovers was approximately 36 hours, four and a half eight-hour shifts, with a standard deviation of 16 hours.
Due to the large variation in completion time, the organization was frequently either: a) understaffed or unable to start the machine on time; or b) overstaffed and waiting to start the machine. Further, the long changeover times necessitated campaigning the different drug products produced on this line. This resulted in larger inventories.
A preliminary goal was established to reduce the overall changeover time to 18 hours (2.25 shifts), a 50% reduction.
Using the Voice of the Customer (VOC) tool, a stakeholder survey tool, the project manager interviewed key stakeholders to establish key requirements for the project. Several themes quickly emerged which helped to properly scope the project:
- Reduce cycle time
- Maintain GMP compliance
- Do not cause “atypical events” that result in investigations or quality reporting
- Do not impact product quality.
- Results must be sustainable and measurable
- Increasing head count is not an option
Because key stakeholders were engaged early in the project design, they were willing to commit the personnel and provide the sponsorship necessary to succeed.
Measure Stage: “What Is Going on in My Process?”
The current state measurement system clearly was not adequate. Calculations of changeover cycle time came from shift change and paper production records—the procedure was time consuming and imprecise. In addition, because data analysis and collection was done away from the floor operations, front-line operators were not aware how changeover cycle time impacted productivity.
The first major change was to have operators measure the changeover times to the nearest hour and post these times on a chart in the area, as they completed each changeover. In addition, the total changeover time was stratified to into three separate time components: 1) tear down; 2) cleaning; 3) setup.
Tear Down Time + Cleaning Time + Setup Time = Total Changeover Cycle Time.
Direct observation and time studies were used to measure the cycle time and generate a process map for each of these time components. This process map charted, step by step, each activity necessary to complete the changeover.
With operator input, these actions were then categorized as; a) value added; b) waste (as defined by the 7 lean wastes); and c) business value added (activities that are non-value adding but necessary for the business, like filling out the batch records).
Examples of “waste” and “business value added” activities for tear down, cleaning, and setup are included in Figure 2.
Next, the team focused on finding out ways to eliminate waste and minimize time spent on business value added activities.
Note: Waste in this case is comprised primarily of motion waste (searching for items) and over-processing (repeated cleaning of tools and clarifying instructions). Business value added activities are primarily documentation related. It is important to recognize that while documentation is important to maintaining regulatory compliance, it is a non-value added activity. Efforts must be made to make documentation as straightforward and mistake proof as possible. This minimizes cycle time variability as document errors and atypical investigations can significantly impact product release and overall cycle times.
Analyze Stage: “How Do I Achieve My Project Goals?”
Based upon previous improvement activities, the area head was used to hearing requests for more labor or resources. If tools weren’t found, new tools were purchased and subsequently misplaced. If documentation was incomplete, more training was implemented. If production couldn’t be met, new equipment, more overtime, or new people were necessary. SOPs became longer and more complex. In this project, those solutions, rightfully, were off the table.
A simple “5 Why’s” analysis was used for each of the identified wastes to develop root causes and brainstorm solutions. An example of a “5 Why” analysis for lost tools is as follows.
During this changeover, tools were frequently lost or misplaced. It was not uncommon for each operator to hoard a private set of tools, often incomplete. While seemingly obvious, setting up and maintaining a common storage location had not been considered.
More importantly, it was clear that the SOP did not document the “best way” to complete the work. Further, it provided too much latitude to the operators. As a result, operators completed the work as they saw fit.
The “5 Why” technique was used for each of the potential wastes to identify root causes. The two most common root causes for the long and highly variable cycle times were:
1. Lack of communication between shifts; and
2. Lack of a standardized work plan.
A SMED (Single Minute Exchange Die) analysis was then used to evaluate each of the activities within each of the time components. SMED is a Lean tool that provides a method to reduce set-up/changeover times. Activities are categorized as internal, external, or non-value-added. Internal activities must be done during the changeover (critical path activities), while external activities are done before the changeover begins. Non-value-added activities are eliminated. Some examples are:
• Internal: Use of spare parts, cart movement, cleaning of the room
• External: Cleaning of tools, movement of parts
• NVA: Filling out the tear-down checklist, loading of multiple racks, searching for materials.
Categorization of activities as NVA and external to the changeover achieved more than 13 hours of cycle time savings.
Improve Stage: “Will These Changes Work?”
In order to improve the changeover process, solutions that addressed the lack of communication between shifts and the lack of a standardized work plan were brainstormed for each of the time components: tear down, cleaning, and setup.
In particular, standardized work, a Lean tool, was used to clearly define each operator’s actions. Standardized work defines each operators actions and the order in which they need to be done.
With standard work, the team was able to synchronize the actions of multiple operators, ensuring that work is completed in a highly efficient and reproducible manner. Standard work reduces variability and thus maximizes safety, quality, and productivity.
Other solutions that were implemented were:
• Kanbans to improve component availability and communication of shortages
• Revision of documentation to eliminate unnecessary paperwork
• Reorganization and elimination of bottleneck steps.
Solutions were piloted and root causes validated over several changeovers with dramatic results—total changeover times were reduced to 17.5 hours, a greater than 50% reduction. Risks were evaluated and showed no possible risk to the product or GMP.
Business impact was calculated and met sponsor targets both in terms of dollar savings and ability to support the market. As procedures were better defined, there was no negative impact to GMP compliance; local SOPs were updated to incorporate the changes in paperwork. The reduction in variability and improvement in performance were achieved with little implementation risk—and at no increased capital cost to the business! The change was thus fully supported by the compliance and quality organizations.
Control Stage: “How Do I Keep These Gains?”
Improvement projects are often able to meet their goals, at least initially; however, performance can degrade with time. In order to fight departmental atrophy, we trained area management in SPC, or Statistical Process Control.
SPC utilizes statistical methods to monitor process performance. This tool helps management detect when process performance begins to “drift.”
A manual SPC control chart was implemented as part of the changeover project and currently resides on the visual board of the operating area. Operators were trained to update and maintain cycle time metrics after each changeover and encouraged to provide suggestions for improvements to supervisors.
About the AuthorJames Lu is as a Founding Partner at GJL Solutions, a Lean Six Sigma/ Operational Excellence consultancy that focuses exclusively on the Pharmaceutical and Biotech Industries.