PharmTech, Inc. (Libertyville, Ill.) recently conducted a survey of representatives of 42 different pharma firms (53 participants in all) about their efforts in supply chain serialization and traceability—in four specific areas: Awareness, Readiness, Business Value and Challenges to Implementation.
The following (including charts) is some of that data, reprinted with permission. For more information, contact PharmTech’s Michael Stewart at [email protected].
Most companies (roughly 70%) were aware of the California e-Pedigree and serialization bill and the guidance by FDA (some 80%) on standardized numerical identifiers.
Awareness of international legislation, however, was mixed, PharmTech notes.
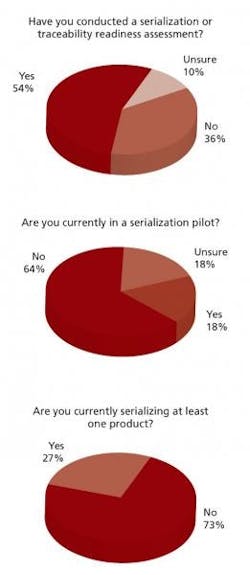
Participants were also asked to estimate the implementation time for a companywide typical serialization and traceability project.
Sixty percent expected the timeline to be one year or less, though PharmTech estimates that, in actuality, it can take 3-5 years depending on the number of lines and products involved,” PharmTech’s report notes.
The greatest organizational challenges for serialization and traceability, according to the participants, were regulatory uncertainty, equipment capital expenditures, and the fact that traceability was viewed as a “compliance only” issue.