Under pressure: Can the glass packaging industry withstand the weight of COVID-19?
It was all smiles at the White House in 2017 when executives from Corning Incorporated visited President Trump’s administration to unveil an innovative new pharmaceutical vial. As part of the White House’s “Made in America” initiative, Corning brought a sample of its Valor Glass container, which was developed with Pfizer and Merck, and had become the first new pharma glass composition approved by the U.S. Food and Drug Administration in over 100 years.
To demonstrate Valor Glass’ durability — one of its prime advantages over traditional borosilicate glass — Corning’s CEO, Wendell Weeks, asked the president to break a conventional vial by pressing down on it with a lever. In one quick pop, the borosilicate vial was shattered.
“I’m pretty strong,” the president quipped with a smile.
“You ain’t seen strong yet, brother,” Weeks responded to a room full of laughter.
When Trump pushed his weight onto the lever pressing down on the Valor Glass, the vial didn’t budge.
At the time, it was touted as another example of ingenuity by an American company. Now, a few years later, glass vials — including Valor Glass — have become a critical linchpin in the White House’s efforts to bring an end to the coronavirus outbreak. And the strength, not just of vials, but of the pharmaceutical glass industry as a whole, will be a determining factor in whether or not America — and the rest of the world — can finally bounce back from the pandemic.
In fact, it wasn’t long after drug developers kicked off their mad dash to create a vaccine earlier this year that the world wised up to the additional concern of having enough vials. For the past few months, headlines have blared that there may be a vial shortage coming and the worries have thrust packaging — part of the pharma industry that’s normally behind the scenes — into the public’s glare.
Meanwhile, the glass packaging market has launched into a fervent sprint of its own to ensure that when a vaccine is approved, pharma companies will have a way to deliver it to patients. Like pharma companies developing a vaccine, the U.S. government has also been helping the packaging industry along, pumping millions of dollars into the efforts to quickly ramp up capacity for glass vials.
But as the industry races to produce the needed vials for SARS-CoV-2 vaccines, small- and mid-sized pharma companies looking to package other therapies could be left behind.
While some glass companies are optimistic that they will be able to meet the demand being generated by the coronavirus while keeping up with existing commitments, others admit that the market for glass vials could be in shortage for many months to come.
How can the glass industry keep its supply lines from cracking under the weight of COVID-19?
Glass or no glass?
There’s a kind of confidence your company can emit only when it’s successfully been in business for over a century. In the tight-knit community of glass vial producers, that self-assuredness is well on display.
“Schott is one of the oldest companies in pharma history and is working closely with the top pharma companies globally,” Stefan Marc Schmidt, vice president of global sales and marketing at Schott AG says. “Right from the start of this COVID crisis, we have been in close contact with customers and have been working on scenarios and different ways to be prepared. That’s what we do.”
“We have been surprised that this is an issue in the press,” Stöcker says.
The combined experience of the top titans in glass manufacturing should make the industry well suited to the task of meeting the unprecedented packaging demands for a SARS-CoV-2 vaccine.
Three companies — Schott, Corning and Nipro Pharma Corporation — control the lion’s share of the market for pharmaceutical glass tubing, which is the primary component used to make containers such as vials and syringes. The top two glass tubing manufacturers have been in business for over 100 years each, and difficult barriers to entry, such as the high complexity and cost of manufacturing type 1 borosilicate glass (the most common type of glass on the pharma packaging market), have kept the tubing industry small. Underneath the glass behemoths, there is also a bustling market of converters — companies who turn glass tubing into vials or syringes.
“There are hundreds of converters who turn tubing into vials,” explains Brendan Mosher, vice president and general manager at Corning. “So you have a very consolidated tubing market and then a very fractured converting industry.”
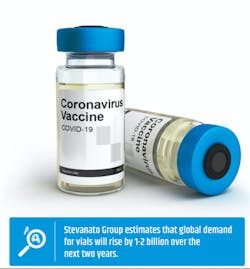
Despite the combined experience of glass manufacturers, with so few of them, it also stands to reason that there could be concerns about the industry being nimble enough to handle a sudden surge in demand. Stevanato Group, an Italian manufacturer of vials, estimates that global demand for vials will rise by 1-2 billion over the next two years. Even before the coronavirus pandemic, the industry faced worries that there could be a type 1 borosilicate glass vial shortage. Part of the problem is that glass manufacturing facilities are expensive to build. Then, there can be challenges with obtaining the needed key raw material — a particular kind of angular sand found in riverbeds and beaches that’s in high demand around the world for a number of products.
Earlier this year, John Bell, a professor at University of Oxford (where one of the leading SARS-CoV-2 vaccine candidates is being developed) sounded the alarm over a potential glass shortage, claiming that there are just 200 million vials left in the world.
But Stöcker waves those concerns away. Based on Schott’s internal auditing of the market, Stöcker says that the current capacity for glass containers for injectable drugs is 50 billion, including cartridges, syringes and other containers. Of that 50 billion, Stöcker says that the current global supply for vials alone is 16-18 billion.
The market was also already on the upswing. Due to rising demand for vials from developing countries, Schmidt says that the glass container market has been expanding 3-5 percent each year, and Schott had already planned a $1 billion investment in building out its existing capacity for both glass tubing production and converting.
“For the last few years…there have been drug shortages in the U.S.,” Stöcker says. “But that has nothing to do with packaging.”
Mosher, however, describes the market for glass tubing as “extremely tight.”
“Corning is the second biggest borosilicate tubing producer behind Schott,” Mosher says. “We make enough tubing for approximately six billion vials a year. But we have been in a sold-out condition for the past 18 months.”
The hunt for glass
Lawrence Ganti, chief business officer at SiO2, a company that has innovated an alternative vial to borosilicate glass, agrees that some pharma companies are having a hard time securing glass packaging from the leading suppliers.
“We have been told by many customers that when they call asking for glass, they are turned away, or asked to pay ridiculous upfronts to obtain it,” he says.
Given the challenging market conditions and the fact that glass manufacturers want to make sure they have enough supplies ready to fill orders for a coronavirus vaccine once one is approved, Ganti says that the top players in “Big Glass” are prioritizing who they can make commitments to — and it’s often only Big Pharma companies.
Currently, Schott says it is not turning down orders or requests from customers.
“We openly discuss the business case, negotiate and make a competitive offer to our customers,” Schmidt says.
But Mosher admits that Corning is sometimes “turning away customers because we have already committed capacity.”
“Big Pharma companies that already have contracts in place should be fine,” Mosher says. “I think for small- to mid-sized companies, it is going to be much more difficult [to secure vials] if they don’t have existing supply agreements.”
How can the glass industry juggle the sudden need for more vials and keep smaller pharma companies stocked up?
Borosilicate alternatives
When SiO2 launched, the goal was to develop a new pharma container that was as stable and safer than glass but as durable as plastic to reduce breakage during manufacturing. After about 10 years and $500 million in R&D, the company released a plastic vial containing a microscopic inner glass lining that’s 50 times thinner than a human hair.
“What we’ve managed is to advance a new material which takes all the durability of plastic and marries that to all of the stability properties of glass so that you have none of the negatives of either but the positives of both,” Ganti says.
Interest in the SiO2 vials has trickled in from pharma companies since the product was commercially released about a year ago — but many customers were reluctant to to take the leap.
“People said it was cool tech but they didn’t want to change because they didn’t want to be the first,” Ganti says.
Now, since a number of Big Pharma companies have converted to the SiO2 platform and there’s more focus on supplying vials for a coronavirus vaccine, Ganti says that the number of companies looking for an alternative vial has shot up.
“About 60 to 70 percent [of pharma companies] are coming to us now because of concerns with glass,” Ganti says. “Before, the time between meetings with companies was months — now it’s days.”
Before 2019, Ganti says that there were seven drug products being tested in clinical trials with SiO2 containers — now there are 41. Given the sudden interest in securing vials, SiO2 has also doubled its sales forecast for this year, from a 200 percent increase over 2019 to a 400-500 percent jump.
Ganti admits that the SiO2 vials are still a bit more expensive (about 5-10 percent more) than traditional borosilicate containers, but stresses the many benefits, such as eliminating breakage, faster time to market and increased patient safety. And because the glass material is made by chemically applying the inner layer — made from SiO2 and vapor — by using plasma energy, there are no raw materials concerns.
Mosher says that Corning is also steering customers in the direction of Valor Glass if the company is unable to fulfill a request for borosilicate containers.
When developing Valor Glass, Corning’s goal was to eliminate delamination from vials, which contributes to glass flaking and subsequent recalls. After conducting a root-cause analysis, Corning discovered that boron — a mineral in borosilicate glass — can become unstable at high temperatures and contribute to delamination. Eventually Corning innovated a new composition that eliminates boron but keeps the glass’ other needed elements: silica and alumina.
The aluminosilicate formulation in Valor Glass not only addresses delamination, it is also more mechanically resistant to temperature swings and is less prone to breaks and damage, which improves efficiency and speed on the filling line.
Mosher says Corning is working with customers every day on adopting Valor Glass for new drugs, but because of the investment associated with revalidating manufacturing lines for new containers, it’s a tougher sell for existing drugs.
“It’s a no-brainer for new drugs,” Mosher says. “But there are time and conversion costs for existing drugs. For that reason, we are working closely with global regulatory authorities to streamline the adoption process for innovative technologies like Valor Glass.”
Even with alternative packaging options on the market, the pharma glass industry is going to have to ramp up capacity for all vials if it’s going avoid being pummeled by rising demand.
A capacity cure?
Just as the Biomedical Advanced Research and Development Authority (BARDA) has been injecting the pharma industry will hundreds of millions of dollars to support the development of a vaccine, government agencies have also been throwing money at the vial problem.
In June, BARDA announced two major investments to expand vial manufacturing — one is a $143 million contract with SiO2 to scale its per-month capacity to 10 million containers (which can hold up to 10 doses of a vaccine each) by November. Ganti says the contract will add 200 employees to its manufacturing site in Alabama. In addition to supplying Moderna with vials for its vaccine candidate, the company is also working with six other coronavirus vaccine developers, who are testing their products in SiO2 containers.
Corning also secured a $204 million grant from BARDA to increase capacity for its Valor Glass products at facilities in New York, New Jersey and North Carolina. Because Corning has agreed to grant priority access to drugmakers designated by BARDA who are developing a vaccine, Mosher says the company could sell out its capacity of Valor Glass to BARDA through 2021.
The Coalition for Epidemic Preparedness Innovations (CEPI) has also invested in securing a supply of vials across the Atlantic. In June, CEPI announced a supply agreement with Stevanato to procure 100 million containers that will be able to hold up to 2 billion doses of a vaccine.
Andrea Zambon, marketing and product management director at Stevanato says that the company has already secured an ample supply of glass containers and is fast-tracking access to four of its converting lines in Italy and Mexico.
“This preferred access [for CEPI] will remain in place through the end of 2021, by which time we will mostly like have a vaccine approved and distributed worldwide,” Zambon says.
When asked if the CEPI deal could impact Stevanato’s ability to keep up with other commitments, Zambon says “not at all.”
“We’ve proactively increased our capacity worldwide, and are committed to maintaining exemplary service to our standing customer base as well as the approximately 30 additional programs in which we’re involved pertaining to COVID-19,” Zambon explains.
The widespread ramp-up mode of pharma is also trickling down to purchases for packaging equipment, according to the Association for Packaging and Process Technologies (PMMI).
“As researchers and scientists actively pursue development of a safe and effective vaccine for the coronavirus, PMMI’s June 2020 Purchasing Index indicates that investments in packaging machinery for the pharmaceutical and medical device sector are vastly exceeding figures for other sectors such as food, beverage and personal care,” says Jorge Izquierdo, vice president, Market Development, PMMI.
Despite the capacity increases, many companies are preparing for a rocky market in the short term.
David Enloe, president and CEO, Aji BioPharma Services, a global integrated drug product contract manufacturer, says his company is now experiencing extended lead times on glass vials that are up to 12 months in some cases — and the situation is likely to get worse before it gets better.
“There is a concern that once effective vaccines become available, lead times may increase even further, despite commitments from glass manufacturers to increase tubing and converting capacity. This will be an added strain on CDMOs and their ability to meet clients’ timelines for non-COVID-19 products,” Enloe says.
To help mitigate the risks, Enloe says Aji BioPharma Services is working with its partners to select vials and stoppers that may be less impacted by the push for a coronavirus vaccine vial.
“For vaccines it appears that a 10mL vial and 20mm serum stopper are most popular, which would provide more than ten doses per vial. There is also interest in 20mL vials with a greater number of doses, which may help cut the vial demand by half,” he says, adding: “It’s important to remember that CDMOs, drug innovators and glass suppliers need to communicate and collaborate now more than ever to address these supply chain challenges in order to successfully address the upcoming COVID-19 vaccine demand as well as continue to support all of the other patient needs.”
How long before the market levels out? The predictions vary.
“In late 2019, it had been predicted that the supply of glass tubing would stabilize by the end of 2020,” says Kazunori Ashida, president of Namicos Corporation, a Japanese manufacturer of ampoule vials. “However, now due to the sudden increase in demand for vials in anticipation of a COVID-19 vaccine rollout, we expect the shortage to continue. For this reason, even though ampoule and vial makers are putting forth strong efforts to ramp up their production capacity, we anticipate that it will take several years to stabilize.”
Mosher says that given the fact that all of the major suppliers have announced capacity expansions, the situation could start to resolve itself “in the next 12 to 18 months.” Mosher adds that the increase in capacity could even eventually lead to an excess in the supply of vials.
One thing glass manufacturers all agree on is that — just like drugmakers — the industry is banding together to make sure there will be enough supply to effectively distribute a coronavirus vaccine around the world.
“The industry is putting aside the competitive spirit,” Mosher says. “It feels like more than ever, that everyone is in this fight together.”