Editor
Within microbiology, a shift is taking place from simple laboratory studies toward greater use of risk assessment and management [1]. Sometimes these approaches form part of a drug company's total quality system, sometimes they exist as standalone techniques. The most important guidelines for pharmaceutical microbiology are described in ICH Q9, including the tools of FMEA (Failure Mode and Effects Analysis); FTA (Fault Tree Analysis); and HACCP (Hazard Analysis Critical Control Points).
The two most commonly used within microbiology are HACCP (which originated in the food industry) and FMEA (which was developed for the engineering industry). This article explores these two approaches, first with a description of HACCP, followed by a description and case study of FMEA in sterility testing.
HACCP: Risk Based Approach in Environmental Monitoring
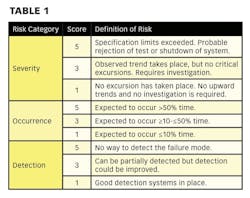
Hazard Analysis and Critical Control Point (HACCP) is a risk assessment approach that addresses physical, chemical, and biological hazards [2]. HACCP is designed so that key actions, known as Critical Control Points (CCPs) can be taken to reduce or eliminate the risk of the hazards being realized. HACCP involves focusing on where the control points in a process are. Once these are established critical limits are set. The critical limits are then monitored and the process is verified as being in control (or not) [3]. There are different variants of HACCP. The “Lifecycle Approach” is similar to that contained in the FDA “Pharmaceutical cGMPs for the 21st Century: A Risk-Based Approach” [4].
There are two key components of HACCP:
• Hazard Analysis: Determining what microbiological, physical, or chemical risks are associated with a process.
• Critical Control Point: A point, step, or procedure at which control can be applied.
In general HACCP involves the following:
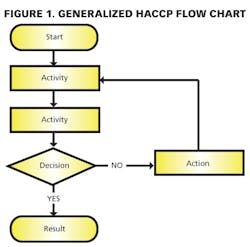
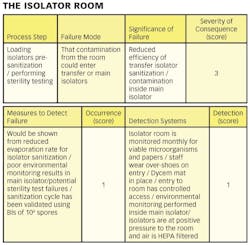
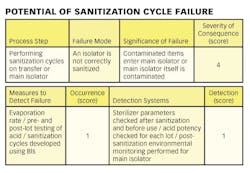
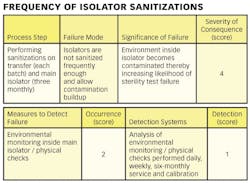
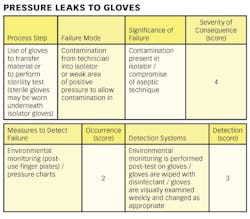
1) Conducting a hazard analysis. This involves listing all potential hazards associated with each step, conduct a hazard analysis, and consider any measures to control identified hazards. For this process flows are useful. For example, see Figure 1.
2) Determining the Critical Control Points (CCPs).
3) Establishing critical limit(s).
4) Establishing a system to monitor control of the CCP.
5) Establishing the corrective action to be taken when monitoring indicates that a particular CCP is not under control.
6) Establishing procedures for verification to confirm that the HACCP system is working effectively.
7) Establishing documentation and record keeping.
References
1. Whyte, W. and Eaton, T. (2004): ‘Microbiological contamination models for use in risk assessment during pharmaceutical production’, European Journal of Parenteral and Pharmaceutical Sciences, Vol. 9, No.1, pp11-15
2. Sidor, L. and Lewus, P. (2007): ‘Using risk analysis in Process Validation’, BioParm International, pp50-57
3. Notermans, S., Barendsz , A. W. and Rombouts, F. (2002): ‘The evolution of microbiological risk assessment in food production’, Foundation Food Micro & Innovation, Netherlands
4. World Health Organisation (2003): Application of Hazard Analysis and Critical Control Point (HACCP) methodology to pharmaceuticals, WHO Technical Report Series No 908, Annex 7, World Health Organisation, Geneva, 2003, link: http://www.who.int/medicines/library/qsm/trs908/trs908-7.pdf
5. De Abreu, C., Pinto, T. and Oliveira, D. (2004): ‘Environmental Monitoring: A Correlation Study Between Viable and Nonviable Particles in Clean Rooms’, Journal of Pharmaceutical Science and Technology, Vol. 58, No.1, January-February 2004, pp45-53
6. The FMEA centre at: http://www.fmeainfocentre.com/introductions.htm
7. Sandle, T. (2003): ‘The use of a risk assessment in the pharmaceutical industry – the application of FMEA to a sterility testing isolator: a case study’, European Journal of Parenteral and Pharmaceutical Sciences, 2003; 8(2): 43-49
8. PDA Technical Report No. 13 (revised): 'Fundamentals of an Environmental Monitoring Programme', September / October 2001
9. EU GMP: 'Rules and Guidance for Pharmaceutical Manufacturers', TSO, UK
10. Farquarharson, G and Whyte, W. (2000): ‘Isolators and Barrier Devices in Pharmaceutical Manufacturing’, PDA Journal of Pharmaceutical Science and Technology, Vol. 54, No.1, January-February 2000, pp33-43
11. Sandle, T. (2004): ‘General Considerations for the Risk Assessment of Isolators used for Aseptic Processes’, Pharmaceutical Manufacturing and Packaging Sourcer, Samedan Ltd, Winter 2004, pp43-47
12. Whipple, A. (1999): ‘Practical validation and monitoring of Isolators used for sterility testing’, European Journal of Parenteral Sciences, 1999, Vol. 4, No.2, pp49-53