Dr. Smith — the development lead for her company’s most promising new drug product — has been losing sleep over the past month pulling together the CMC (Chemistry, Manufacturing and Control) section of the regulatory submission. The compound has shown excellent efficacy and safety data, and the entire company is counting on a quick review and approval. Dr. Smith is now faced with a significant headache, though. She is searching for key information related to solubility that was characterized early in development. She had to email, call and even physically visit the medicinal chemistry team. While she has been successful with most of the data, results from one series of tests is proving hard to find. The individual responsible for the tests long ago left the company and now no one knows where to find the missing information. Dr. Smith knows that re-running the tests would be risky — and might even delay the filing — so she is now in a manual process to hunt down the data across multiple databases.
This is an issue commonly heard across pharma companies. While this specific example occurs in CMC, similar problems happen in many of the other functions. As pharma companies are becoming increasingly lean, new processes and technology approaches are needed to move our scientists, engineers and specialists away from documentation management and back to their core skill set.
NEW APPROACHES AND TOOLS FOR A CHALLENGING LANDSCAPE
Figure 1. Representative Core Drug Product and Process Data across Functions.
Recent years have brought tremendous change to the pharma landscape. Significant economic pressures are driving an increased focus on costs within healthcare systems and payers around the world are under increased pressure to deliver incremental outcomes for patients. This, in turn, is forcing pharma companies to reduce costs and development cycle times, while broadening their portfolios to include specialty products, large and small molecule drugs, biosimilars, generics and combination drug/device products.
Pharma companies employ different models to succeed in this demanding new environment. One common challenge across these models — discovery, development and launch of new products — is management of product and process data. As companies “lean out” and speed up their development processes with new technologies, they face increasing challenges with capturing and efficiently managing data as it flows from their labs to their plants around the globe. This is further complicated as companies leverage external development and manufacturing partnerships for speed and cost and they face the same challenges outside the “four walls” of their process and technology landscape. To address this product and process data challenge, pharma companies are beginning to tap into the capabilities of Product Lifecycle Management (PLM) processes and tools. So, for Dr. Smith, a solution is starting to emerge.
SPECIFIC PRODUCT CORE DATA CHALLENGES
Product and process data define a compound, as the compound moves from discovery through development and, hopefully, onto the market. Today, product and process data is created and managed by multiple functions via independent processes throughout the drug development lifecycle (from discovery through to launch). In most pharma companies, information is mostly stored in documents and usually separated among functions. Some companies have dozens of separate databases and/or systems to collect vast amounts of data. As a result, valuable time is lost in collating information that is required to file with regulatory agencies, respond to regulatory questions/investigations, or revisit a technical or management decision. With the above pressures on reducing time to market while improving development and manufacturing efficiency, an accurate and integrated product and process data structure becomes essential. And the existing manual and resource-intensive approaches will not be tolerated.
Based on our work with top pharma companies, we have identified several common challenges with product and process data management. While the ones listed below are more prevalent in chemistry, manufacturing and controls function, there are other product and process data related challenges within clinical, regulatory, quality, manufacturing, supply chain, safety and commercial. Some of the key CMC challenges include:
• Inadequate linkage between product-related data and their sources slows down product development and tech transfer — Often, there are “multiple sources of truth” and information is manually entered and then re-entered into downstream systems. For example, critical product information captured in electronic laboratory notebooks (eLNs) is used to draft technical reports that are stored in document management systems. Specific technical reports (and sometimes raw data) are then retrieved and manually assembled for decision-making in a collaboration tool. Finally, the approved information is reformatted into a larger document such as one for technology transfer, where it is then manually re-keyed into manufacturing or supply chain systems. These manual processes not only involve multiple rounds of wasted time on clerical work, but they are also prone to errors that can ultimately prove detrimental to the product in terms of time-to-market or approved label.
• Document-centric approach detracts from the ability to support timely regulatory submissions and the re-use of information across products — Typically, key product information is buried in documents and it is difficult to access, maintain and re-use. In addition to the challenges of data re-entry, a document-centric approach prevents teams from re-using the cumulative knowledge across products or technology platforms. We have even seen cases where experiments had to be re-run years later, simply due to the company’s inability to find the original set of results.
• Lack of integrated workflow execution forces the organization to rely on fleeting “tribal” knowledge — Often, changes to one set of critical product data are not systematically reviewed, approved and communicated to all parties involved. For example, a change in the toxicology profile of a common excipient can impact multiple products and their specifications or impurity profiles, but critical information communicated via e-mail regarding a single material or product is often not leveraged across the portfolio. And, even if it is, this is driven by key individuals and not systematic within the company.
• Poor project visibility limits the ability to make rapid program decisions — Data availability is inadequate to make program decisions quickly, as information about project status is locked in function-specific documents and can differ based on system or site. Project tasks and deliverables are manually tracked — with no clear linkage between a task, its deliverable(s) and the corresponding product structure — and require manual update for status and completion.
• Labor intensive internal and external collaboration prevents bidirectional innovation — Exchange of information with third party organizations also often happens via emails. This information typically requires additional steps to import, store and manage. As a result, the focus is on management of isolated documents and information and not on the collaborative review and evaluation of that information, for product and process improvements, or effective response to events.
THE RISE OF PLM IN PHARMA
Figure 2. PLM for Pharma Maturity Model.
To address these challenges, pharma companies are looking at a variety of solutions. These include enhanced semantic search capabilities, utilization of collaboration tools and more rigorous approaches to Master Data Management. But one of the more intriguing options emerging is to adopt and modify PLM practices that have proven successful in other industries.
PLM is the business capability of leveraging product data throughout a product’s lifecycle in order to gain efficiency from a single source of accurate, complete and timely product-focused data. Modern PLM is critical — and widely adopted — in the electronics, automobile and aerospace/defense industries. More recently, it has also become prevalent in food/beverage, consumer packaged goods and the medical devices industries. Key benefits include:
• Use of data across products, to improve time to market and support portfolio analytics
• Ability to manage product information (structure, requirements, project plans, changes) along the end-to-end product lifecycle, across core commercialization functions
• A secure collaboration platform for internal and external co-development, review of changes and resolution of adverse events or deviations
• Automation of administrative tasks, enabling scientists and technical experts to focus on their core roles instead of data search, information retrieval, or document writing
• Re-use of existing data for product variation management to further accelerate time to market
To begin to understand the value of PLM in pharma, companies have started by asking a critical question: what are the core drug product and process data in a pharma setting? While companies are tackling this question from various functional perspectives, we believe that core drug product and process data is truly cross-functional in nature — and must be created and consumed across multiple functions including clinical, CMC, regulatory, quality, manufacturing, supply chain, safety and commercial. Figure 1 depicts some of the representative core drug product and process data created, maintained and consumed by these functions.
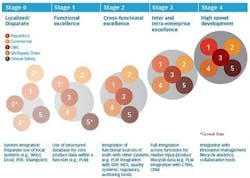
How are core drug product and process data handled by these various functions? While some pre-commercial functions such as clinical have more experience managing data to comply with Good Clinical Practices (GCP), other functions do not. Furthermore, each function has tended to advance its product and process data management capabilities independently to serve their own purposes, as they transfer documents and data during development and technical transfer to commercial operations. We refer to this progression of product and process data management capabilities as the “PLM for Pharma Maturity Model,” as depicted in figure 2.
Stage 0 depicts immature product and process data management where product information is captured in documents, without a standard format and stored in local systems. The information sharing across functions is highly manual, and scientists or managers waste a lot of time looking for information, creating reports and often copying data from one place to another. This is largely the status-quo of product and process data management in the pharma industry, in functions such as CMC, quality and supply chain.
On the other hand, some functions are more advanced in their data management capabilities today, driven by the absolute need to capture, re-use and correlate product data. An example is the clinical function, where fairly mature structured databases (e.g., Electronic Data Capture (EDC) and Clinical Trial Management System (CTMS)) are in place to consistently capture data in a standard format. This enables easy search and analysis of large sets of data. We see some pharma companies leveraging functionally focused capabilities to advance to the next stage of maturity, Stage 1. In this stage, we see standardization of data structures, transition from document-centric to data-centric models and consolidation of data into one single source of truth, albeit in one or a few functions. These outcomes can be enabled by traditional PLM solutions.
Figure 3. Vision of a Mature Stage 2 PLM Maturity in a pharma company.
However, drugs cannot be developed and manufactured in a siloed manner. Functions must share data in an integrated manner in order to efficiently progress through the development, launch and commercial product lifecycle. This need brings us to Stage 2 of PLM for pharma maturity. A typical integration point for sharing of core product and process data occurs between PLM (e.g., CMC-derived data), Manufacturing Execution System (MES) (e.g., manufacturing process data) and Enterprise Resource Planning (ERP) (e.g., supply chain-related data). This integration facilitates a smooth tech transfer and can accurately track product disposition. Another example includes the integration of core product and process data derived from CMC with regulatory information management systems, so that the burden of creating and validating regulatory submission documents can be reduced. Figure 2 represents one pharma company’s vision of a mature Stage 2 PLM model (with plans to achieve it during the next 3 years).
Stage 3 of maturity is achieved when product and process data management is integrated across all the internal development and commercialization functions and with external partners. This is where marketing data or competitive information can inform the target product profiles in real-time and product development can become more adaptive and flexible. Tight integration across functions also allows creation of the full Common Technical Document (CTD) or similar dossier submissions, including potential vendor participation and ultimately accelerates the timeline to filing. Data Lake and semantic technologies are tools that can enable the full integration.
Finally, Stage 4 represents the future of PLM for pharma, where historical and real-time information, including non-conforming data and hypothesis across functions, are synthesized to inform adaptive and predictive design of a product. This typically involves advanced collaboration, analytical and cognitive tools across the integrated and logical data flow to achieve high-speed product development throughput. This stage is still aspirational for most pharma companies, but is effectively used by leading companies outside of our industry.
PLM CAN MAKE A DIFFERENCE
As the pharma industry strives to achieve increasing efficiency in response to global economic challenges, new capabilities such as product and process data management, collaboration and analytics are needed. Some leading technology providers are tooling their traditional PLM solutions — broadly used in other industries — to meet pharma’s needs, the specific requirements of integrating product and process data. Pharma companies should think strategically about the best ways to adopt these capabilities, carefully select the most appropriate solutions and then design creative deployments to make a set of solutions co-exist across various functions. Increasingly, pharma companies are turning to PLM to address these challenges, but they should be thoughtful about its design and implementation, as the “right” architecture for pharma may look familiar in places but very different in others, when compared to other industries.