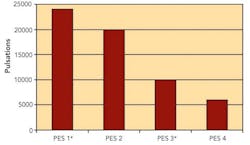
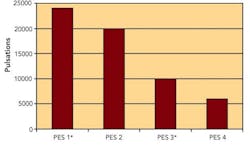
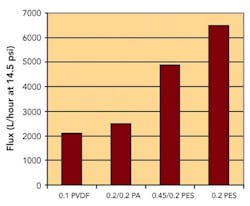
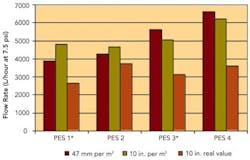
Sterilizing Filters: Right Flow, Right Size Critical
By Jennifer Maynard, Six Sigma Black Belt, Baxter BioScience; Theodore H. Meltzer, Capitola Consultancy; Maik W. Jornitz, vice president, Product Management, Sartorius North America, Inc.; and Paul M. Priebe, head of Product Management, Process Filtration, Sartorius North America, Inc.Sterilizing grade, 0.2-micron-rated membrane filters are used widely within the biopharmaceutical industry. They were also given more prominence by FDA’s Aseptic Guidelines, which specified filtrative sterilization as a key criterion for process validation (www.pharmamanufacturing.com/industrynews/2004/93.html).Originally used in cases where thermal sterilization would degrade product, the filters have been redesigned and optimized to improve flow. They are now recognized as the quickest, most cost-effective means to achieve sterilization for large volumes of simple buffers or aqueous solutions.Since such fluids typically contain only very low levels of contaminants, a sterilizing filter’s throughput is far less important than its speed in transferring fluid from a mixing vessel to either holding vessels or disposable bag containment. The longer the transfer takes, the longer equipment will be idle, directly affecting the manufacturing facility’s capacity.Consider, for example, a common 0.2-micron-rated sterilizing-grade filter, which commonly achieves a flow rate of 2,500 L/hour/14.5 psi. It would take this filter 48 minutes to process a 2,000-L volume. In contrast, an optimized high-flow-rate filter with a flow rate of 6,000 L/hour/14.5 psi would require only 20 minutes, doubling equipment availability. In cases where that speed would be too high, it could be adjusted by reducing the filter’s effective filtration area (EFA).Where, in the past, sterilizing-grade filters were designed to be used broadly for a multitude of applications and fluids, they are now customized for specific applications, some of which require a high flow rate through the filter at low differential pressure. This article will discuss the importance of flow within these applications, focusing on how best to test the filters for flow and how to size them accordingly.Improved designsRedesigning the filters’ membranes and cartridges allowed them to achieve higher flow rates. Otherwise, the only way to improve flow rate would have been to raise the differential pressure or increase surface area, neither of which was practical: higher differential pressures would mean a significant increase in energy consumption or could risk exceeding the filter’s operating pressure capacity. Larger filtration surfaces would mean an increase in consumable and capital investment costs.
About the AuthorsJennifer Maynard is a Six Sigma Black Belt for Baxter BioScience in Thousand Oaks, Calif., currently working within purchasing supplier management. She has extensive knowledge in supply chain management and process improvement within cell culture manufacturing.Theodore H. Meltzer, Ph.D., is a private consultant with Capitola Consultancy in Bethesda, Md., with over 40 years experience in the separation technology and ultrapure water industry. He is the author and co-author of multiple books, book chapters and over 100 scientific papers.Paul M. Priebe is head of product management — process filtration, for Sartorius North America (Edgewood, N.Y.) He has more than 8 years experience in process filtration. He has previously held positions in manufacturing, engineering, sales and product management.Maik W. Jornitz is VP of product management at Sartorius. Jornitz supports the biopharmaceutical industry with close to 20 years of experience in separation technologies. He is author and co-author of multiple books, book chapters and scientific publications.References1. ASTM Committee F-21. Standard Test Methods for determining Retention of Membrane Filters Utilized for Liquid Filtration. Annual Book ASTM Stand., Philadelphia, Pa., 1998; 790–795.2. FDA, CDER. Guidance for Industry, Changes to an Approved NDA or ANDA, CMC, 1999.3. HIMA. Microbial Evaluation of Filters for Sterilizing Liquids.Health Industry Manufacturers Association, Washington, D.C., 1982; Document No. 3, Vol. 4.4. Jornitz, M.W.; Meltzer, T.H. Flow and Pressure, Flow Decay, and Filter Sizing, and Cartridges, Cartridge Holders, and Their Care. In Sterile Filtration – A Practical Approach, Jornitz, M.W.; Meltzer, T.H., Eds; Marcel Dekker, New York, N.Y., 2001; Chapters 4 and 8.5. Levy, R.V. Sterile Filtration of Liquids and Gasses. In Disinfection, Sterilization, and Preservation; Block, S.S., Ed.; Lippincott Williams & Wilkins, Philadelphia, Pa., 2001; Chapter 40.6. PDA. Sterilizing Filtration of Liquids, Technical Report #26, PDA J. Pharm. Sci. Technol. Suppl. 1998; Vol. 52, No. S1.7. Soelkner. P.; Rupp. J. Cartridge Filters. In Filtration on the Biopharmaceutical Industry. Meltzer, T.H., Jornitz, M.W., Eds. Marcel Dekker, New York, N.Y., 1998.8. Priebe, P.M.; Jornitz, M.W.; Meltzer, T.H. Making an Informed Membrane Filter Choice – Criteria to Consider. Bioprocess Int. 2003, 10, 64–66.9. Jornitz, M.W.; Soelkner, P.G.; Meltzer, T.H. The Economics of Modern Sterile Filtration. Pharm. Technol. U.S. 2003, 27(3), 156–166.10. Jornitz, M.W.; Meltzer, T.H., Bromm, H. and Priebe, P.M. Choosing the Appropriate Membrane Filter - Test Requirements, PDA Journal, Vol. 59, No. 2, 2005, 96 - 102