In the biopharmaceutical industry, companies are increasingly deciding to implement disposable flow paths that can help amplify the flexibility of their standalone and/or multi-product facilities. In addition, to help their customers ease this transition, suppliers have taken their expertise in hardware design engineering, process engineering coupled with aseptic filtration, mixing dynamics and film technology to deliver disposable solutions that meet the required flexibility needs.
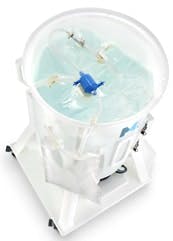
The Mobius MIX200 Disposable Mixing System includes a 200 L carrier, a single-use magnetically driven impeller inside a disposable Mobius process container and an electronic drive unit.
Despite the fact that stainless steel equipment continues to be recognized as a standard offering in the industry, single-use solutions offer a more flexible approach. Found within a range of applications, single-use solutions are quickly becoming a critical component in manufacturing. These solutions are utilized in both upstream and downstream processes, and often involve using single-use process containers, assemblies complete with tubing and fittings, as well as separation tools for virus removal and other purification processes.
Over the past decade, companies have found that single-use technologies offer a solution to many of their manufacturing plights. While single-use solutions are beneficial in process development, implementing disposable technologies doesn’t immediately eliminate the use of stainless steel components. With the struggle to undergo a transitional period in order to meet the growing needs of single-use devices, the biopharmaceutical industry is having difficulty with integration. Customers are looking towards hybrid solutions that can integrate with or replace traditional stainless steel hardware. Suppliers have to respond to these evolving needs by offering a range of flexible products.
Next-generation processes require tools that steadily meet customer requirements of speed, flexibility and cost-effectiveness. A number of solutions already exist that are helping to make the transition from stainless steel to disposable options easier for manufacturers.
Mixing Technologies
The need for integration has driven today’s disposable products to mimic the design of traditional stainless steel tanks. As a result, existing mixing technologies are designed with round or conical process containers, which are notably similar to stainless steel tanks that have tulip-shaped bottoms.
In addition, various devices employ a magnetically driven impeller, which is located inside a disposable process container. Mixing is achieved by the action of the impeller’s conversion to energy, exacting the combination of flow and turbulence to achieve adequate mixing. In order to attain sufficient dissolution or mixing, the ratio of liquid to the vessel diameter is maintained to provide consistent flow. As a result, the position of the impeller eliminates staged flow patterns, which further strengthens the 1:1 ratio. This not only decreases the risk of contamination, but it also makes it possible to quickly reproduce the process.
Disposable Process Containers
Every company in the biopharmaceutical industry, regardless of how well-known it may be, is rushing to get its drug to the finish line. With such elevated competition, it becomes inefficient to spend large amounts of time on cleaning validation.
While stainless steel equipment has to be closely monitored for contamination, disposable process containers reduce this downtime. These next-generation tools aim to provide ease of use and flexibility for the customer. Made of high-purity, multilayer medical-grade film, disposable process containers have low extractable levels that are ideal for bioprocessing applications such as media, buffer preparation, process intermediates, bulk storage and transportation of biopharmaceutical fluids in sterile conditions.
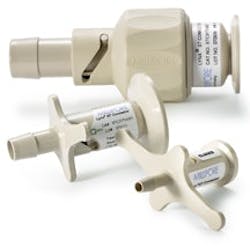
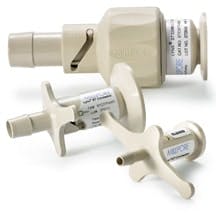
Lynx connectors, made of USP Class VI polysulfone, provide secure connections between sterilized fluid paths.
Valves and Connectors
Valves and connectors are additional next-generation components that are regularly used with disposable process containers. Designed to connect pre-sterilized assemblies to processing systems, connectors eliminate improper sterilization, as there are no parts to clean-in-place.
Unlike aseptic components that leave room for contamination to seep through, disposable connectors ensure that sterile fluid is securely transferred between two assemblies. Disposable components therefore provide a cost-effective and rapid solution for achieving maximum results.
Integrated Solutions
The evolution of disposable technologies has provided manufacturers with an integrated approach for designing efficient and economical processes when scaling a product from clinical development to commercialization.
What’s more, suppliers offer pre-assembled disposable solutions that integrate individual disposable components into a complete system. Filters, tubing, process containers and connectors can all be assembled, packaged and sterilized before being sent to the customer.
Conclusion
The ability to seamlessly integrate single-use technologies into existing processes has allowed manufacturers to operate more efficiently. Specific benefits include greater process flexibility, cost savings due to reduced labor for cleaning and validation, and increased speed to market and clinic.
With the biopharmaceutical industry expanding progressively, it is becoming even more crucial for companies to maintain a competitive edge. Disposable technology not only eliminates of the amount of time spent on cleaning validation, but also speeds time to market. Integrating stainless steel equipment and disposable technologies gives biopharma manufacturers additional opportunities to optimize their processes from upstream to downstream.