Demand for Innovation: Disposable Devices Top the List in 2011
Oct. 11, 2011
12 min read
The demand for improvements in single use, disposable bioprocessing equipment have shot to the top of end-users’ wish list of better, more innovative products in 2011. And the industry’s push for better single use purification devices leads the pack with almost 40% of our 352 survey respondents this year looking for better downstream single use products. [1] The data, released in BioPlan Associates' 8th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, points to an increasing concern that innovations in disposable bioprocessing products have been slow to appear. Our annual global study, which had responses from biomanufacturers in 31 countries, identified many ‘problems in need of solutions.’
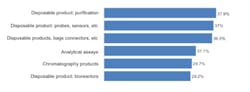
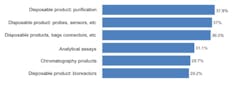
We asked respondents to identify their top areas where suppliers should focus their development efforts. Among the 21 most commonly cited areas, improved single use/disposable products led, with four of the top six areas. Single use purification products, probes/sensors and bags/liners, connectors, and bioreactors were among the contenders for the next new biomanufacturing products. We found, for example, that 29% of the industry desired improvements in bioreactors, generally one of the largest bioprocessing expenses. The type of bioreactors also largely determines other up- and down-stream equipment selections. In comparison to disposable equipment, only 6% indicated a desire for improvements in fixed stainless steel bioprocessing equipment. Figure 1: Selected New Product Development Focus Areas, Where Suppliers Must Focus Development Efforts, 2011Source: 8th Annual Report and Survey of Biopharmaceutical Manufacturing, April 2011, BioPlan Associates, Inc. www.bioplanassociates.com We also evaluated the trend in demands for new products from vendors, and found some significant shifts. Interest in better innovation in disposable, single-use devices for measuring and monitoring (probes, sensors, etc) jumped from 29.3% of respondents in 2010 to 37.0% this year. Relatively few of these products are currently available and there are often issues regarding ports and how to pass disposable sensors through bioreactors and other vessels and their bag liners. Meanwhile, improved disposable purification products, which led all areas this year at 37.9% of respondents, showed modest growth from last year’s 34.9%. While this was the area where users most desired improved single-use products, the result was not a surprise. Improvements in upstream manufacturing have greatly enhanced yields in recent years, largely as a result of improved cell lines and expression systems. However, downstream purification processes have scarcely changed and are increasingly the limiting factor in commercial-scale biopharmaceutical manufacture. Also, purification, including chromatography columns and media, with different steps and equipment, can be time-consuming and expensive. Interest in disposable bags, connectors, and other such products modestly decreased from 2010 (36.5% this year, compared to 38.9% in 2010), but remains high on the list of desired innovations. Possible innovations such as unitary (single-piece) molded plastic bioreactors could offer advantages, including in performance and reduced costs, over adding expensive plastic bags/liners to expensive stainless steel bioreactors and other containers. However, the predominant single-use paradigm continues to be adding multi-layer plastic laminate bags/ liners to what are essentially classic-design stainless steel bioreactors, mixers and other fluid containers. Many of the key vendors are committed to this approach, and some have recently invested in costly bag-making manufacturing facilities. These companies will be dedicated to current product lines and this paradigm for years to come. The problem with this approach, along with a host of other current single-use equipment designs, is that they curb opportunities for innovation and new product introductions. Once initiated, vendors are effectively unable to significantly upgrade existing product lines. Furthermore, incremental product upgrades risk seriously distressing their existing customers, particularly if current products are phased out. Indeed, bioprocessing product changes require considerable expense and work, including modification of regulatory filings, SOPs and training, and perhaps most discouragingly, potentially requiring another round of validation testing. The approximately $100,000 price tag for testing of devices with significant product contact (required for approvals) stands as a major deterrent. Interest in assays grew significantly this year, from 24.5% of respondents in 2010 to 31.1%, in line with a trend expectation from the industry, which sees more and more automated assays being implemented to achieve higher quality and consistency.[1] However, some of last year’s key new product areas declined in interest among respondents. These include: chromatography products (37% down to 30%) and process development, both downstream and upstream (35% to 27% and 25% to 19%, respectively). This lessening of concern for new bioreactor products may be related to the emergence of some interesting innovations, which have sparked recognitions such as PBS Biotech’s recent honoring as Business of the Year by Pacific Coast Business Times. In particular, small scale single use bioreactors, with traditional impeller designs like NBS or Millipore’s, have begun to fill some of the gaps in the disposable bioreactor market. This is an example whereby the available “bag design” bioreactors did not meet customers’ needs, and a creative alternative emerged. In some business models and specific situations, small scale bioreactors will replace bag design. Even so, according to Robert Repetto, Director of Technology and Innovation at Pfizer, “it will not revolutionize the bioreactor market, as its scope is too limited to be disruptive to the industry.”[1] Additional areas of general interest to respondents, both this year and last, where increased attention to new product development was desired, include:
- Online monitoring and control
- Improved quality and consistency of materials
- Energy efficiency
- Quality control and consistency
- Reliability/robustness of analytical equipment
- Instrument accuracy and reliability.
Source: 8th Annual Report and Survey of Biopharmaceutical Manufacturing, April 2011, BioPlan Associates, Inc. www.bioplanassociates.com For the most part, respondents’ attitudes remained steady in comparison to 2010. The area that experienced the largest shift was operations staff training, which dropped 13%, from 60% in 2010 to 47% this year. Other areas that showed the largest shifts from 2010 were optimized media and improved existing quality management systems (QMS). On the other hand, overall better control of process, use of disposable/single use devices and improved upstream production operations all decreased by a modest margin from 2010. CMOs and biotherapeutic manufacturers differed considerably in their responses. CMOs overwhelmingly reported performance improvements from use of disposable/single-use devices, at an astounding 86% (compared to 63% of biomanufacturers). Surprisingly, developers reported more frequent implementation of process-related improvements. For example, 68.4% of biomanufacturers signaled that better process development had contributed to improvements, compared to 58.6% of CMOs. This is somewhat unexpected, given that CMOs have a high project turnover and are under constant pressure to get things right the first time. However, it may relate to increased FDA concerns and fallout from major pharmaceutical manufacturing failures such as Genzyme, which have put them under the spotlight. By contrast, there have been few widely publicized CMO failures, and their focus on smaller-scale manufacturing leaves them less prone to strict regulatory requirements.In comparing responses by region, the use of single-use devices was cited for improvements more often by the US and Western Europe, with just 55% of “Rest of World” countries attributing improvements to this factor. This may reflect European customer purchases or vendor marketing catching up with those in the US, while much of the ROW may not yet be heavily implementing single-use systems. Looking AheadThe industry this year and next should see a number of innovations in single use devices, from bags, to bioreactors, to tubing and connectors. Many of these products will be targeted to replace silicone, including PVDF (inert Teflon-like fluorinated polymer)-lined tubing and polypropylene-PVDF and other thermoplastic blend tubing. Materials-based innovations and new, improved, product designs enabled by these are needed for single-use bioprocessing to become more cost-effective and to support single-use/disposable equipment being adopted for manufacture of commercial products. Beyond these projected advances, few highly innovative products, such as commercial-scale perfusion bioreactors and new single-use bioreactor designs with ≥2,000 L capacity, have been introduced, and this seems unlikely to change in the near term. There seems to be broad recognition that the development of new materials, particularly new and improved plastics and variations of current plastics that might enable major design innovations, are needed. Yet, the major players that dominate the single-use/disposable bioprocessing systems market have committed to current product lines for at least a few years. In the meantime, they may see little incentive for introducing paradigm-shifting product lines. Innovations in single-use equipment, particularly in novel technical devices such as bioreactors, may instead be more likely to originate from small companies or new major corporate entrants. Many respondents’ wishes for improved single-use equipment may in fact represent a desire for decreased cost of this equipment, along with obvious improvements in productivity. This may open the door for lower cost producers, or those able to provide more functionality, quality, or validation support at the same price.References:1. 8th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production: A Survey of Biotherapeutic Developers and Contract Manufacturing Organizations, BioPlan Associates, April 2011, 490 pages.2. See BioPlan’s Top 1000 Global Biopharmaceutical Facilities Index™, http://www.top1000bio.com/index.asp Accessed August 20, 2011About the Author: Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD in 1989. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing”, and many other industry reports. [email protected] 301-921-5979. www.bioplanassociates.comSurvey Methodology: The 2011 eighth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production in the series of annual evaluations by BioPlan Associates, Inc. yields a composite view and trend analysis from 352 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 31 countries. The methodology also encompassed an additional 186 direct suppliers of materials, services and equipment to this industry. This year's survey covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world's major markets in the U.S. and Europe.
About the Author
Eric S. Langer
President
Sign up for our eNewsletters
Get the latest news and updates