Every technical (tech) transfer is different, with companies taking different paths toward reaching their goal. Each one presents a unique set of challenges, which are most often technical, but at times may also be business related. However, all donors (outsourcing partners) and recipients (contract manufacturers) have the same overall goal — to achieve a quick, efficient transfer of process and knowledge that meets all necessary quality and regulatory requirements.
Alkermes Contract Pharma Services engages with a diverse range of donor partners on contract manufacturing. From internal transfers within Alkermes’ own development pipeline, to specialty companies with one key product in their pipeline requiring transfer, to “Big Pharma” where any individual tech transfer is one of a large portfolio of diverse transfers taking place at one time. We have found that donor companies have differing approaches to tech transfer, ranging from those who wish to be involved in every step of the process, to those who take a much more hands-off approach. Our approach is to fulfil each of their specific needs and satisfy their requirements as effectively as possible.
To help drive the tech transfer of partner products, Alkermes sought to develop a risk assessment framework that involved both the donor company and our transfer team in jointly assessing the tech transfer risks, which would address not just the usual technical challenges but also, where appropriate, the business challenges.
User Requirements for a Joint Risk Assessment Framework
The user requirements in the development of this framework were defined as follows:
- The framework had to meet regulatory guidelines on process transfer and development (ICH Q8) and follow the industry direction on risk-based process development/ transfer.
- The framework had to be effective and efficient, contributing to a quick and effective transfer.
- The framework had to be easy to use, avoiding long, bureaucratic processes and facilitating easy participation for the partner.
The aim of the joint risk assessment process was to provide a framework with the following outputs:
- Provide Alkermes and the donor with a strong, combined assessment of the risks inherent in the tech transfer.
- Identify any potential gaps or unacceptable risks in the tech transfer plan and facilitate risk mitigation/elimination.
- Identify scope of engineering studies required (if any).
- Identify any risks that may remain open on moving into the upstream stages of the transfer including clinical supply, registration, process validation, and eventually, commercial supply.
- Clarify the owner of each risk, ensuring that both sides are aware of and are in agreement on the risks.
Meeting Regulatory Requirements
As covered in the ICH Q8 Pharmaceutical Development Guidelines, the core source document for the risk assessment process is the Quality Target Product Profile. In many tech transfers, this profile is communicated to the receiving site via the donor site product quality specifications. This document identifies the Critical Quality Attributes (CQA) of the process to be transferred.
All risks assessed as part of the risk assessment are examined for their impact against the product CQAs. As the success of a tech transfer is measured not only by the quality of the product produced, but also by the efficiency of the process, each risk is also assessed against Key Manufacturability Attributes (KMAs) such as process repeatability and robustness.
Making the Framework Effective
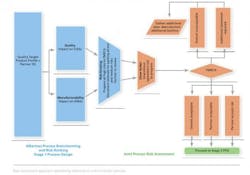
The approach taken by Alkermes is a Quality Risk Management approach based on proven standard risk assessment tools. First the risks are identified via brainstorming. The risks are then analyzed and evaluated via a risk ranking process and the use of Failure Mode, Effects and Criticality Analysis (FMECA). As part of this process, the joint team (of donor and recipient members) starts to consider the controls that should be put in place or the additional data and/or studies required to mitigate the risks identified.
Before starting to risk assess, two critical pieces of the puzzle must be in place:
- The correct team must be assembled, comprising the technical, quality, validation and, if necessary, regulatory personnel, from both the donor and recipient sites. It is vital that these are the personnel who will be responsible for the activities throughout the tech transfer and on into clinical and/or commercial supply. All team members must be present from the start of the process.
- The structure and approach of the joint risk assessment must be agreed upon with the donor in advance. This includes the agreement of all definitions, e.g., assumptions, boundaries, high-risk ranking, critical severity, remote possibility, etc. Clear, unambiguous definition of these terms at the outset will greatly aid the efficiency of the process throughout.
Making the Framework Easy to Use
To enhance the ease of use of the joint risk assessment framework, some sub-processes were added on to classic Quality Risk Management tools.
Initially brainstorming and risk-ranking processes are performed with only the recipient team present. All risks that are suggested are included in the listing process and progressed for ranking. The risk-ranking process assesses whether the risk identified can directly impact a CQA or KMA.
By completing this portion with the recipient team first, the donor team is not required to sit through the initial debates on ranking. The provisionally ranked listing is then presented to the donor, and the donor’s input is managed by exception, i.e., the joint team only debates any rankings with which the donor does not agree. Using this approach, we typically find that the joint risk-ranking process can be completed within one working day, even for complex multi-unit operations. Our experience also indicates that this meeting should, where possible, be conducted face-to-face and attended by the full team on both sides. At this early stage, full participation is invaluable to building an understanding of each other’s systems and approaches.
The cut-off for progressing items from the risk ranking to the FMECA stage will have been agreed upon before commencing the risk assessment. Typically, all risks ranked as “high risk” will require full FMECA. The risk-ranking process does not assess the probability or severity of a risk occurring or if there are strong controls already in place.
These assessments take place at the FMECA stage. However, particularly for new processes, there are risks that must be ranked high as they directly impact a CQA, but which usually fall down the relative risk ranking when severity, probability or existing control review is applied. A recent example was the risk associated with a power outage during a fluid bed drying cycle on a machine. While a power outage would be a significant risk, the fact that the drying cycle machine had back-up power rendered it a low-risk activity.
To reduce the time associated with working risks through the full FMECA process, we have introduced a sub-process between risk ranking and the FMECA stage referred to as the “silver bullet” step. Here, unanimous agreement of both teams can render the risk acceptable based on existing knowledge. It is important that the justification for applying the silver bullet be thoroughly documented.
Three Paths to Process Quality
To further improve the efficiency of the process, multiple decision-point criteria are provided to allow the FMECA to be brought to closure. Defined in advance, these decision-point criteria allow the FMECA process to be concluded and a recommendation made as to the appropriateness of moving forward to the next stage of the tech transfer. For example, in the case study that is described below (Section B), three paths to proceed to the process qualification stage were agreed to with the donor:
1. Donor and recipient agree that the risk is acceptable.
2. Donor and recipient agree that more data is required. Donor has existing supporting data. Donor determines that risk is acceptable and takes responsibility for the risk.
3. Recipient deems the risk is unacceptable based on limited experience. Donor is prepared to accept risk.
Such decision paths are sometimes required due to the disparity in the process history knowledge between the donor and recipient sites. The key here is that the risk is considered, documented and responsibility for the risk is clearly agreed upon while allowing an efficient closure of the process via a simple declaration by the donor. Such decisions must always be made with the quality of the product and safety of patients as the primary consideration, regardless of the source of the process history knowledge.
IncludE Business Risks
While the joint risk assessment typically addresses technical risks, business or project risks that can potentially impact the tech transfer may also be included. For example, where time or material constraints are impacting the extent of engineering trials that can be performed, a risk may be included to reflect this situation. Allowing for the inclusion of such business risks ensures that both the donor and recipient have a shared understanding of the risks and are moving ahead on an agreed basis.
The inclusion of such risks may also help clarify where areas of process responsibility reside. For example, the donor may be responsible for the quality and delivery of the active ingredient, while the recipient site may be responsible for the sourcing of excipient(s).
Risk Assessment Iterations
It is important to note that the risk assessment process is iterative and is repeated at multiple points during the tech transfer. The initial assessment, including first pass FMECA, is completed after an initial data review when the donor has provided the transfer data pack and the recipient has built a first draft of the process design. These documents are used to generate a list of all possible risks to the transfer. At this point, the list can be extensive with both parties working through a process of identifying risk with a view to eliminating all risks or mitigating all risks. The objective is that no risks remain in the high-risk category as the project moves into the process qualification or registration stage.
In identifying risk controls, the objective is to identify the engineering studies, equipment upgrades and other controls required to execute the tech transfer. Depending on the complexity of the engineering studies required, the next round of risk assessment is performed at the end of the engineering campaign or at logical breaks in a larger, more complex engineering campaign. At this point, the previously identified risks are reviewed to see if they have been eliminated or reduced by the outputs of the engineering campaign. Any new risks identified during the engineering phase are also assessed. The output of this round of risk assessment allows the transfer to move forward to demonstration phase or alternatively may outline what further engineering trials or controls are required before moving on.
At the demonstration batch stage, a demonstration batch is typically manufactured employing the quality systems, facility, equipment, documentation, etc., proposed for use in the process qualification, registration or clinical supply stage, thereby test-driving the process being transferred. On completion of the demonstration batch, the final round of risk assessment is performed. Again, this round of risk assessment aims to conclude that all risks are at an acceptable level and the tech transfer may move into the process qualification or registration phase.
At Alkermes, the various rounds of risk assessments are linked into our project management system and act as important inputs into the technical reviews conducted at key project stages such as End of Engineering and Ready for Validation (and/or Registration, as appropriate) points.
Lessons Learned, Next Steps
Alkermes has found the framework described here very valuable in executing efficient tech transfers and has received favorable feedback from their partners on its use. The framework is now integrated with, and flows into, the three-stage risk assessment process that forms part of the implementation of the new Process Validation Guidance issued by the FDA in January 2011 – Stage 1, Process Development risk assessment, Stage 2, Process Performance Qualification (PPQ) risk assessment and Stage 3, Continuous Process Verification (CPV) risk assessment. This process is led by the receiving site’s validation group.
Lessons learned include:
- Donor companies find it very reassuring to have a fully documented set of risks and responsibilities.
- By agreeing on the mechanism/areas of responsibility upfront, issues during transfer can be quickly resolved.
- Improved upfront detailing of assumptions that lie behind the risk assessment can reduce the number of risks that are dealt with via the silver bullet mechanism and thus reduce the time associated with their assessment.
- Many clients have their own donor-side tech transfer risk assessments. By agreeing on the mechanisms and definitions at the start of the tech transfer, the Alkermes risk assessment has been used to “kill two birds with one stone” with the client taking or referencing the joint risk assessment in their internal systems.
- Involving all stakeholders from both sides from the outset is invaluable. The process and any differences in the processes between the donor and recipient sites are well understood long before the critical process qualification stage. This can make the review and approval of validation protocols, master batch records and change controls much more efficient.
- Dedicating time to the risk assessment is key — the first time a new process, piece of equipment or a commercial partner is involved in a risk assessment is time consuming. Subsequent transfers can leverage the risk assessments developed in previous transfers. Eventually, a library of risk assessments covering all key unit operations is built.
Existing Alkermes equipment was to be employed for one core unit operation. This required a process scale change from the donor site.
A lean approach to the tech transfer was agreed upon, i.e., only one active engineering batch was to be manufactured prior to process validation.
The regulatory filings were the responsibility of the donor company. Minimal development data or product history was shared with the recipient site.
3. The Approach – Joint Risk Assessment
The first round of risk assessment was performed based on the client’s transfer plan and process knowledge at Alkermes.
It was agreed that all risks identified must be closed out by one of the following means prior to proceeding to process validation:
- Donor and Alkermes deem the risks to be acceptable.
- Donor and Alkermes deem additional data is required. Donor to provide/facilitate data gathering.
- Donor and Alkermes deem additional data is required. Donor has existing supporting data. Donor declares the risk is acceptable.
- Alkermes deems the risk is unacceptable. Donor prepared to accept risk.
- Donor and Alkermes deem the risk to be unacceptable. Further work is required to close out.
4. Risks identified
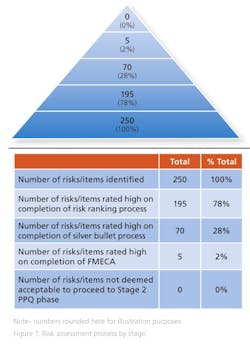
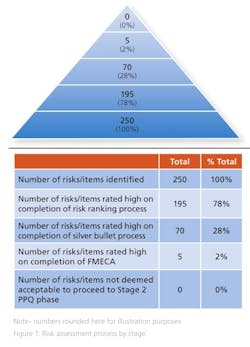
Some 250 risks were identified at the initial brainstorming session and presented to the donor. Of these, 195 were deemed to be high risk, as they directly impacted a CQA at the end of the risk ranking process.
On completion of the risk ranking and silver bullet process with the donor company, 70 risks retained a high-risk designation and were progressed to FMECA. These risks included the risks associated with the single active engineering batch approach. The FMECA process identified the engineering studies required. This included a short placebo manufacturing campaign to support the lean active engineering campaign.
The risk assessment was revisited and updated at the end of the placebo engineering trials, i.e., before the active engineering demonstration batch and again on completion of the demonstration batch. At this point, zero technical and five business risks remained identified as high risk. All quality risks had been mitigated via the additional engineering studies and controls identified through the various iterations of the risk assessment. The remaining business risks were all deemed acceptable by the donor as they related to process historical data readily available to the donor but not shared with the Alkermes team. It was assessed, agreed and documented that the five business risks had no quality or patient safety impact (Figure 1). It was agreed to progress to validation (Stage 2 PPQ) on this basis.
5. Conclusion – Successful Risk Assessment Completion
The time committed to jointly risk assessing the process played a lead role in the timely and successful tech transfer and validation of this product. The framework helped focus both parties throughout the tech transfer and was revisited many times to assist in technical decision-making. The work put into the risk assessment also fed directly into the development of the validation strategy for the product in question. The tech transfer was deemed to be a success by both parties.
The framework was demonstrated to meet the user requirements suggested early in its development — it supported both parties in meeting their ICH obligations on risk assessment in tech transfers and confirmed the effectiveness of Alkermes’ QRM approach. The transfer was deemed to be efficient, and the framework was found to be easy to use.
Alkermes’ approach to risk assessment has evolved since the framework was first developed. These developments and the integration of the joint risk assessment with the continuous validation risk assessment approach will be discussed in the second article in this series.
In the coming months, the Alkermes Validation Group will publish a second article in this publication in which the further evolution of this approach and its integration with the continuous validation risk structures within Alkermes will be charted.
Published in the February 2013 issue of Pharmaceutical Manufacturing magazine.