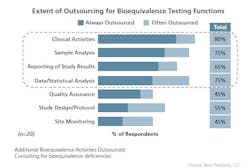
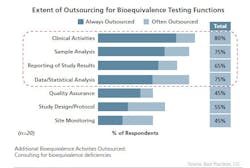
The generics drug market has grown substantially over the last several years and this expansion is expected to continue. Generic companies are increasing the level of Abbreviated New Drug Application (ANDA) filings and in support, adopting a business model that increasingly relies on outsourcing to optimize efficiencies and contain costs. As a business model, outsourcing has strategic and competitive benefits well recognized by the generic pharmaceutical industry processors and suppliers that comprise the bulk of the industry. For instance, the outsourcing of bioequivalence studies and the manufacture of active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs) is widely practiced. An evaluation from Best Practices LLC on “Pharma Bioequivalence Strategies: Performance Metrics, Processes and Trends” provides recent insights on the outsourcing of bioequivalence (BE) studies by generic companies. According to the study, a majority of generic companies queried outsource facets of their bioequivalence studies to less-regulated regions, such as India, China, Russia and Eastern Europe, with India being the preferred outsourcing destination and China and Russia being the less commonly used outsourcing destinations. Outsourcing bioequivalence has its challenges, particularly regarding all matters concerning FDA Quality Assurance requirements and regulatory issues. Despite these challenges, outsourcing is pervasive: commonly outsourced activities include clinical and sample analysis, results reporting and data and statistical analysis. The most frequently outsourced bioequivalence function is clinical activity, according to the Best Practices report. All of the benchmarked Generic companies in the study responded that they outsource clinical activities at least some of the time and 65% always do (see chart on following page).Outsourcing activities also commonly include working with contract research organizations (CROs), investigators and clinical sites, which may or may not be in a contract relationship with the CROs. The selection of a CRO is based on a best-practice selection process involving bids from more than one CRO and framed so that outsourced activities are generally contractually delineated with deliverables on a set time frame and the ability to terminate the outsourcing relationship.Outsourced activities are amenable to auditing and generic companies audit the CROs, investigators and clinical sites frequently, with the majority of the audits occurring before the bioequivalence studies begin. Multiple deciding factors are followed to select which studies need on-site monitoring. Common factors often include whether or not a study is pilot or pivotal, possesses certain “uniqueness” factors and previous study experience with the CRO.QA Kept CloseQuality assurance, which is a critical step in the bioequivalence study process, is seldom outsourced. Most generic companies house a dedicated and centralized clinical quality assurance (CQA) function and for good reason. The CQA function plays a key role in pre-study assessment of clinical sites and the CROs and is viewed as an essential component in improving bioequivalence study quality, reducing deficiency letters, accelerating the review process and improving the relationship with the FDA.Maintaining a regulatory department is a critical element of the study process and, similar to QA, is seldom outsourced. The regulatory department plays a key role in ensuring that documentation is complete and up to date to ensure necessary repeat analysis of clinical, medical and analytical studies; internal regulatory compliance for acceptance of bioequivalence data in support of ANDA; and to promote regulatory transparency.To ensure excellence, as well as cost containment and efficiencies, while managing the risk, partner selection is a critical process and the key to bioequivalence outsourcing excellence. Efficient organizations have in place best-practice procedures for monitoring the outsourcing partner and the progress of the bioequivalence studies underway. Vigilance, due diligence and communication create an atmosphere of success and will always support the effective management of these relationships.Selection criteria should be carefully considered — standards that focus on solid business success and reputation, as well as financial stability are important, as is a demonstrated expertise in bioequivalence study execution. Does the CRO provide a dedicated team knowledgeable in drug characteristics biology and pharmaceutics? Does it have established, successful relationships with key regulatory agencies?When it comes to managing a bioequivalence study outsourcing relationship effectively, best practices include setting up standard operating procedures and conducting pre- and post-trial audits. Correspondingly, on-site monitoring and regular quality assessments for different groups involved in the bioequivalence study are elementary as well. Finally, maintaining strict compliance regimes while maintaining good communication between the various organizational facets is fundamental to winning value from an outsourcing partnership. Best practices in the progress of the bioequivalence study include instituting efficiencies in the testing itself, by optimizing the use of the outsourcing partner through negotiating agreements that save time and money in terms of utilization by experiment and optimization of employee productivity.Risk management strategies that include holding the outsourcing partner to higher quality standards in terms of managing documentation will always serve the interests of both parties. It’s understood that with bioequivalence studies, complete documentation is essential to ensure the necessary repeat analysis of clinical, medical and analytical studies. It is also essential for the ability to convert from paper to electronic formats, and for regulatory compliance and transparency and FDA filings. Contributing as well to bioequivalence study success is the active involvement of the generic company outside of the relationship, with the CROs active in the design of pilot studies to improve design of pivotal studies and faster screening of experimental formulations; design and FDA acceptance of the protocols are also key components.
Enter GDUFAThe Generic Drug User Fee Act of 2012 (GDUFA) is a newcomer and is likely to have an impact on costs associated with the outsourcing of manufacturing of materials. While GDUFA is designed to speed access to safe and effective generic drugs to the public and reduce costs to industry, it requires that domestic and foreign FDF and API manufacturing facilities be inspected and pay user fees. GDUFA is likely to require the generic company to audit their outsourcing partners as the higher probability of an FDA inspection will mean a greater risk of supply interruption due to compliance failures. The increased inspection activity is likely to reduce the numbers of outsourcing partners and raise prices, which are likely to be passed to the generic company. However, it is equally likely that the quality and consistency of APIs and FDFs will improve, which would benefit generic companies.Because GDUFA requires the payment of user fees, which are lower for domestic manufacturing facilities than for foreign manufacturing facilities, these costs are likely to be passed on to the generic company. It is expected that generic companies will continue to outsource bioequivalence studies and manufacturing of APIs and FDFs. In order to maximize efficiencies and cost containment from outsourcing, generic companies need to evaluate their in-house activities versus their outsourcing activities. Generic companies also need to carefully select their outsourcing partner, holding both core competencies and cost containment as key components. Following selection, instituting best practices to successfully manage the outsourcing relationship are key factors for outsourcing excellence.Published in the June 2013 issue of Pharmaceutical Manufacturing magazine
About the Author
Lina Genovesi
Ph.D.
Sign up for our eNewsletters
Get the latest news and updates