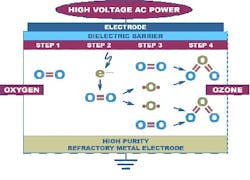
Access to clean water is one of the most important factors considered in selection and development of a new manufacturing facility. Without readily available appropriately clean water, processing would be nearly impossible, changeover and cleaning of reusable equipment would cease, and overall production would grind to a stop.For example, in the United States, incoming water into any manufacturing facility must meet a minimum standard of EPA drinking water. This municipally treated water is only a first step to the processing required by the pharmaceutical industry to bring this water up to pharmacopoeia standards for Purified Water (PW) per USP <1231>. Commonly used technologies to manufacture PW and other compendial waters include softening, reverse osmosis (RO), deionization (DI), dechlorination, (ultra)filtration and even distillation.A comprehensive water management plan must address all the water in the facility, including its treatment, storage, delivery and handling. Part of this control plan requires understanding the needs for both periodic and continuous sanitization to mitigate and prevent the buildup of contaminants such as biofilm within the water system.What is ozone and what does it do?Among the alternatives available for water sanitization, ozone is recognized as an excellent option for disinfecting biopharmaceutical water systems. Ozone (O3) is a highly reactive molecule made up of three atoms of oxygen, which decays back to oxygen. Care must be taken because ozone is also a toxic gas characterized by a strong, pungent smell.Ozone occurs naturally but can be generated. The two most common generation methods for pharmaceutical use include silent discharge generation and electrolytic generation. Electrolytic ozone is created when high voltage passes between parallel metal electrodes and through a liquid containing oxygen, e.g., the process water. Electrolytically generated ozone is often used for small quantities of ozone or lower flows. Ozone generated by silent discharge is created when a current-controlled electric discharge is released between high purity refractory metal electrodes into a gas containing oxygen. The ozone is then injected into the water system. Ozone generated by silent discharge provides greater flexibility in terms of controlling ozone concentrations to the amount needed, as it is only limited by the oxygen content of the air rather than by the oxygen usable from the water.The two methods have different maintenance requirements. Silent discharge generators do not directly touch the water path and generally require little standard maintenance. Electrolytic generators are in direct contact with the process water and will require an isolation system to remove it from the water flow for service. Maintenance recommendations from the manufacturers should be followed to provide the greatest uptime possible.Prior industry concern is that ozone — specifically that generated by silent discharge — may be considered an “added substance,” as it is introduced into the process water vs. created from it. According to the "added substances" discussion in USP <1231>, every added substance must be removed with appropriate means. As long as it is removed, ozone is not considered an added substance in the production of pharmaceutical grade water. Ozone is often removed via a destruct mechanism such as 254-nm ultraviolet (UV) light.As ozone is the same no matter how it is generated, with the same standard CAS Registry Number 10028-15-6, and because, for risk mitigation, most ozone systems already have a UV destruct included, this “added substance” concern is already addressed and can be considered slightly outdated. High purity silent discharge generation ozone systems are available with excellent operating history in other highly demanding industries (e.g., semiconductor) and are the standard for mitigating the high levels of contamination in wastewater treatment.
Figure 1: Ozone manufactured by silent discharge methodOzone Addresses SanitizationOzone is used as an alternative to heat sanitization with hot water and steam or chemical disinfection using chlorine, chlorides, peroxides and other chemicals. It is one of the strongest commercially available oxidants, with a disinfecting strength 3,000 times that of chlorine due to its high eV potential. Ozone effectively kills bacteria, viruses, yeast, fungi and other microbes as a function of time, susceptibility of the target organisms (action), ozone concentration and water temperature.While there are a number of potential sources of contamination in storage and delivery systems for purified and sterile water, one of the most common problems facing purified water (PW) production and delivery is the prevention and removal of biofilms. First described in the mid-1930s, biofilms form on any surface wherever surface-associated microbes are present. Biofilms occur in a wide variety of systems, but are endemic to water systems in the biopharmaceutical industry.Biofilms are difficult to remove from the wetted surfaces of PW systems for several reasons. The most prevalent of these reasons is the tough surface polymeric exopolysacharrides (EPS) shell biofilms excrete. The EPS provides a matrix in which nutrients are retained and microbes can thrive, thereby continuously contaminating the PW storage and distribution system. Biofilm removal treatments must not only penetrate the EPS but must also be biocidal to the heterogenous microbial population below it. Partial removal of the biofilm’s EPS matrix liberates the underlying colonizing microbes. Unless destroyed, these underlying microbes will migrate and reestablish at new sites, maintaining the contamination of the water system. Addressing biofilms is thus critical for the maintenance of high-purity water quality. Periodic and continuous sanitizations are options to mitigate biofilm growth and proliferation. However, targeted destruction of biofilms is difficult as there are not many non-specific treatments that can address biofilms’ inherent compositional variability.Many industrial settings use strong oxidant chemistries such as chlorine and peroxides for biofilm mitigation and removal. Recently, however, drawbacks with these conventional approaches such as water contamination concerns, tightening environmental regulations and chemical costs have led different industries to explore the use of ozonated water for the removal of biofilms and destruction of microbial contamination.As a non-specific biocide, ozone reacts rapidly with most hydrocarbons to effectively destroy the biofilms’ EPS, and the microbes and organic residue material within these films.
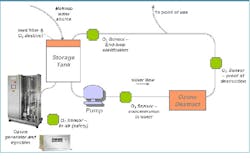
Figure 2: A diagram of specific design considerations for ozone-based sanitation systemsOzone Systems DesignOzone is generated on-demand, in ambient water temperatures, allowing production of ambient temperature PW. Ozone systems for pharmaceutical PW production require ozone generation; injection and mixing into water (if with silent discharge), or mixing alone (if with electrolytic ozone); testing both the initial dissolved ozone output and ozone return; and residual ozone destruction via a UV or other destruct system. Where a storage vessel is used, storage vessel configuration, venting and vent gas destruction may be required. Specific design considerations for ozone based sanitization systems include dissolved ozone sensor location for initial ozone production and to confirm destruction; placing a near-generator ozone gas monitor for environmental monitoring and worker safety; and considering the system’s materials of construction. Ozone dissociates back to oxygen, so oxygen-sensitive materials may be affected by the use of high or prolonged amounts of ozone. This effect should be considered during pre-design risk assessments and feasibility.Configurable systems are available offering two or more of the critical ozone systems components integrated and fully tested prior to installation on-site. There are also systems available which include most of these components in a single package, offering ease of installation and use.Determining the amount of ozone needed for your system depends on the efficiency of the ozone dissolution into the water, the amount of water in the water storage tank and piping distribution, the make-up water quality, and flow rates and pressures, including turnover time. Ozone generators themselves may include requirements for power, clean oxygen-containing feed gas such as compressed air, water cooling and pressure. Finally, as with any standard critical water system, system design considerations of automation capabilities, power, water flow, water temperature control, and pressure must be taken into account. Monitoring and control parameters for safety, flow rate, pressure, and all compendial water purity requirements including pH and TOC should be included in the system to ensure compliance with the monographs.Feasibility ConsiderationsIn the pharmaceutical industry, ozone can be used for general sanitization, for specific tool/system cleaning (clean-in-place, or CIP), or as part of a resolution plan to resolve a specific contamination incident. ASTM standard E2500 removed a number of impediments to the implementation of ozone-based purification in pharmaceutical manufacturing, encouraging improvements in Process Analytical Technology (PAT) through well-documented, robust and flexible manufacturing capabilities. While ozone is very useful, it may not always be feasible for a company’s system. To use ozone, the following parameters must be assessed as part of a risk management evaluation:• Process sensitivity to oxygen;• Materials of construction, and • Operator and environmental safety and procedures around useRegarding process sensitivity, the water system may experience a potential slight increase in CO2 , O2 and trace nitrates. These may not be noticeable, as with trace (ppb) levels of organics are already in water. Per the pharmacopoeia, PW, HPW (Highly Purified Water) and Water for Injection (WFI) are allowed to have specific low levels of ionic impurities. Ozone destruction must be confirmed for processing use and added substances. As ozone dissociates back to oxygen, some oxygen-sensitive materials of construction may be affected. Any potential ozone-wetted components including piping, pumps, seals/gaskets, valve seats, filters and sensors should be assessed and compared with manufacturers’ standards to understand compatibility with ozone. Some examples of materials that are compatible with ozone include PVDF, 316L stainless, and PTFE and Viton.Operator and environmental safety and procedures are also an important consideration. Ozone is a toxic gas in high quantities or concentrations, and is regulated by OSHA in the United States and similar agencies worldwide. For safety purposes, gaseous ozone detection monitors are often placed directly near ozone generators or in the rooms in which they operate to provide early detection should a leak occur. Appropriate venting or destruction of unused ozone gas is important to maintain a safe working environment. Finally, environmental regulations must also be considered when venting ozone gas outside the facility and/or disposing of ozonated water. Ozone can remain in ambient water for approximately 20 minutes before completely reverting to oxygen.Finally, as for any critical water system, a comprehensive risk analysis should be performed. Robust risk mitigation plans including monitoring, preventative maintenance, backup considerations, spares and response flow checklists should be developed. Mitigation plans also consider equipment protection. Common ways to protect the ozone equipment and water system from contamination include water trapping devices, to avoid generator contamination; water level monitors; and automatic shut-off valves. Ozone generator suppliers should be able to provide results proving thorough testing and high quality, to help a company ensure it is installing a robust system and has appropriate support if and when required.All water systems, including ozone-based systems, should be integrated with facilities’ safety and monitoring systems and automated where appropriate for control, monitoring, and troubleshooting purposes.Potential to SaveOzone is both safe and economical to use since it can be reliably generated on-site as needed, avoiding handling and costs associated with strong oxidant transportation and storage. It is generated at ambient temperature and is soluble in ambient temperature water, increasing ease of operation. The infrastructure requirements for thermochemical sterilization and subsequent decontamination are significant, and the use of ozonated water can greatly reduce capital, operations and maintenance costs of water treatment. Ozonated water leaves no chemical residues, unlike other chemical sanitization procedures, and in ambient water reverts back to oxygen naturally. Therefore, it does not need to be flushed as does chemically sanitized water.Any model for ozone savings would compare against baseline system sanitization methodologies, water use and electricity use. Baseline costs include operating costs, energy consumption, utility requirements, heating, steam, cooling waste water and chemical waste, as well as the cost to store, handle and manage chemicals if they are used. The comprehensive cost of water must also be included, for processing, cleaning and sanitization.Once the baseline is developed, the capital costs of ozone are calculated for the required specific components (e.g., ozone generators, ozone monitors and destruct systems). Changes in power consumption, net water usage, maintenance cost and time, possible reduction in sanitization time and frequency and increased asset utilization for existing systems — to “do more with less” — can support a business case. Additionally, if a company is investigating expansion at an existing facility, it may also be possible to avoid capital outlay for an expensive hot water-sanitized loop if hot water is not expressly required by the process.Depending on water usage and needs, facilities can experience rapid payback periods. Additional financial benefits can come from achieving municipal, corporate and facility goals for reduced water use. For example, LEED (Leadership in Energy and Environmental Design), an ecology-oriented building certification program run under the U.S. Green Building Council (USGBC) provides water efficiency credits, which can support a company’s or facility’s sustainability goals and translate into financial benefits.The recent ISPE Critical Utilities conference in February 2013 highlighted the many savings attributable to ozone. Case studies were presented showing daily and annual savings for new builds and even after conversion from non-ozone sanitizing methodologies. Further references are available in ISPE Good Practice Guide entitled “Ozone Sanitization of Pharmaceutical Water Systems.”Ozone is a viable and accepted technological option for water sanitization and one of the most effective technologies against biofilm mitigation. It’s rapidly increasing popularity and acceptance as a solution for PW disinfection is due to its cost-savings potential, including its ability to save significant time during the cleaning cycle. Total cost of ownership models for ozone should include not only its upfront capital costs, but also overall lower or reduced annual maintenance costs, increased uptime available to the facility from both shortened cleaning times, and the possibility for shorter and/or less frequent sanitizations due to improved water quality and reliability. The upside potential of increased number of batches, plus benefits from long-term goals such as LEED-recommended water reduction may also be included in cost/benefit calculations. Ozone can help address the “4 Rs” of water use in a pharmaceutical facility: reduce, reuse, recycle and reclaim. Using ozone at ambient temperature for sanitization will reduce net wastewater and electricity used in hot water and chemical sanitization. Without the addition of chemicals, ozone can help reuse critical water within the facility, and if the capabilities are available, can support reclaiming water (effluent) coming out of the plant. With its proven results against biofilm, ozone systems are a credible sanitization option for water expansions, greenfield sites and conversion from existing hot water or chemical systems. Ozone technology is available and its benefits readily quantifiable so should be closely considered as a feasible option for future critical water needs.Published in the June 2013 edition of Pharmaceutical Manufacturing magazine
References:
•ISPE Baseline Pharmaceutical Engineering Guide for New and Renovated Facilities: Volume 4, Water and Steam Systems, Second Edition, December 2011
•ISPE Good Practice Guide, Ozone Sanitization of Pharmaceutical Water Systems, July 2012
•ASTM Standard E2500, 2012, “Standard Guide for Specification, Design and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment”, ASTM International, West Conshohocken, PA, DOI: 10.1520/E2500-07R12, www.astm.org
•ISPE Knowledge Brief KB0022-June10: “Ozone Sanitized Pharmaceutical Water Systems: Tank Venting Concerns”
•A.K. Greene, Z.P. Guzel-Seydim and A.C. Seydim, “Chemical and Physical Properties of Ozone” in Ozone in Food Processing, Colim O’Donnell, B.K. Tiwari, P.J. Cullen and R. G. Rice - Editors, John Wiley and Sons, Oxford, UK (2012) pp 19-31.
•M.W. Mittelman, “Biofilm development in purified water systems”, in Microbial Biofilms, H. M. Lappin-Scott and J. William Costerton, Eds., Cambridge University Press, Cambridge, UK, (1995) pp 133-147.
•Samuel Stucki, Dirk Schulze, Dieter Schuster, and Christian Stark “Ozonation of Purified Water Systems” in Pharmaceutical Engineering, January/February 2005, Vol. 25, No. 1
•Joe Manfredi, “Cost Optimization Alternatives for Critical Utilities”, presentation at the ISPE Critical Utilities conference, February 2013
•Nissan Cohen, “Economic Efficacy Comparison Between Ozone and Hot water Sanitization” , presentation at the ISPE Critical Utilities conference, February 2013
About the Author
Erika Hanley-Onken
MKS Instruments Inc.
Sign up for our eNewsletters
Get the latest news and updates