Maximizing Uptime for Mission-Critical Manufacturing Units
Manufacturing drugs, particularly biopharmaceuticals, is a mission-critical business demanding continuous uptime. Loss of power or other utilities can have impacts that reach far beyond the plant floor and the individual batch, affecting patients and investors, and damaging corporate and brand reputation.
In R&D facilities, a single power outage can destroy months or even years of experimental data. On the manufacturing and production side, a power loss can compromise product integrity, threaten vital sanitation standards and potentially jeopardize a pharmaceutical manufacturers ability to meet stringent government regulatory requirements.
Currently, the industrys capacity utilization rate is said to average around 33%. However, this might be the wrong factor to focus on. In fact, what is really needed is an uptime-focused engineering design process. Taking this approach would allow operational reliability studies to be conducted early in the design process, to maximize facility effectiveness and enhance the value of capital investment. It would also allow cost/benefit strategies to be developed early on, to ensure the reliability of a plants mechanical and electrical systems.
Maintaining maximum operational continuity should not only be seen as a strategic business goal, but also as an essential to any facilitys or companys survival. Executives and facility operators continually must ask themselves: How much would my company lose in revenue if our manufacturing facility lost production capability for a day? An hour? A few minutes?
This article will touch on some of the design, construction and maintenance issues affecting mission-critical drug manufacturing facilities, offering some best practices and opportunities for adding value.
The heating/ventilation/air conditioning (HVAC) areas are typically the largest and most energy-intensive parts of any drug manufacturing plant. HVAC and power systems must be designed for as close to 100% uptime as possible, taking into consideration both scheduled maintenance and unplanned power failures. With the annual cost of energy approaching the installation cost of the mechanical/electrical systems, even slight improvements in energy efficiency mean significant operating cost savings.
DOE and EPA data have shown that energy efficiency costs can be very quickly recouped (see "Improving Energy Efficiency in Pharmaceutical Manufacturing Operations"). Therefore, any time spent considering energy efficiency improvement options is likely to pay off. Computer-based building performance simulation tools can be extremely effective weapons in the war against downtime. They offer a way to evaluate system redundancy options and optimize the relative reliabilities of different power and mechanical system alternatives.
Pharmaceutical Design Challenges
Operational effectiveness and environmental sterility are key design factors for any pharmaceutical facility. Early focus on addressing manufacturing requirements is critical to maximizing the value of the capital investment. Good engineering increases value by improving productivity, minimizing downtime and reducing the cost of goods.
Controlled, ultra-clean environments are required for aseptic manufacturing, but they often require high water consumption levels, and heat loads approaching 100 watts per square foot. Economic design strategies are essential to meeting requirements while improving efficiency. Various codes and regulations define the environmental cleanliness levels and measures of particle concentrations, temperature and humidity required for specific processing steps. Air filtration, air circulation rates, robust cleaning and controlled operational procedures all must support the different cleanliness levels required. At cleanroom entries, for example, air pressure cascades drive air and particle movement towards lower classification areas to maintain environmental control.
All systems must be designed to support commissioning and validation activities, and to facilitate rapid restart after unplanned outages. Using installation inspection, commissioning and maintenance documentation can support validation activities, saving time and money during project implementation, and improving facility reliability and performance thereafter.
Pharmaceutical projects require precise documentation to meet regulatory requirements and to justify capital costs. Documenting specific client requirements is essential to understanding and fulfilling them effectively. Balancing cost expenditures in proportion to benefits derived helps identify the best choice from many design options.
Overstating quality requirements and tolerances may result in unnecessary costs. Consider the existing utility infrastructure closely, and take full advantage of any synergies. Higher air flows and pressures require more HVAC capacity. Since most engineering decisions will have an impact on HVAC systems, it is important to recognize opportunities to seek the best engineering solutions.
The Three Rs and the Value of N
Pharmaceutical manufacturing facility designs should emphasize operational effectiveness, minimize downtime and allow for quick recovery after unplanned outages. The concept of the 3Rs redundancy, reliability and recovery must be incorporated throughout the design process: reliable products with a proven track record must be provided, redundant equipment and systems must be installed, and facilities must be designed for quick recovery after unplanned outages.
It is also critical to determine the degree of redundancy required to ensure reliability. Any additional costs for back-up chillers or other equipment must be weighed against the value of lost production to determine the proper level of redundancy required.
Pharmaceutical manufacturing facilities can have very high internal heat loads. The most energy intensive equipment includes air handling units, water-cooled chillers, cooling towers, chilled and heating water systems, pure water systems, steam systems and other process equipment. These facilities must be designed to execute efficient commissioning and validation activities. The systems must operate as designed and facility operators must be able to restart systems, or allow the facility to recover after unplanned outages. Manufacturing personnel need accurate as-built documentation to enable troubleshooting and system analyses. This documentation also facilitates the development of routine preventative maintenance programs.
Pharmaceutical Life Cycle Enhancements
Good engineering fulfills stakeholder requirements while maximizing value to the company. Saving initial costs is possible through reduced infrastructure from integrated design strategies and equipment right sizing. Facilities designed with this approach demonstrate significant life cycle cost savings. Critical decisions during the design process can increase architectural efficiency, energy efficiency and operational efficiency for pharmaceutical facilities.
Increasing life cycle value through improved equipment usually increases capital costs. Premium efficiency motors and variable frequency drives (VFDs) can increase capital costs, but most pharmaceutical companies find that those costs are recuperated quickly because the technologies reduce operating costs and facilitate good engineering decisions day to day, on the manufacturing plant floor. Environmental considerations often create opportunities for value enhancements that may not be obvious.
Pharmaceutical air handling systems support clean aseptic environments, so filtration efficiency is a critical design consideration. Sealed, no-bypass air filtration systems often cost more than traditional systems, but lead to longer equipment life, increased High Efficiency Particulate Air (HEPA) filter life and lower overall maintenance costs.
Manufacturing facilities whose water supplies are exposed to salt air must have an effective filtration system to prevent damage to any sensitive electronics in the facility. The use of enhanced filtration systems can improve filter efficiencies dramatically, allowing lower grade filters to be used to achieve the same cleanliness levels, with substantial savings. In coastal climates, using copper instead of aluminum for air handler coil fins requires higher initial capital costs, but offers improved durability.
Higher investment costs are typically recouped many times over when the initial cost evaluation factors in the cost of degraded thermal performance, eventual coil replacement and lost productivity associated with resultant downtime. Designs that simultaneously save capital costs and life cycle costs are best found by incorporating efficiency at all levels and integrating the savings into other systems.
For pharmaceutical manufacturing facilities, the greatest enhancements in life cycle value often come from improvements in operational efficiency. Pumping systems, for example, work more efficiently with fewer elbows and larger pipe sizing. Designs that minimize elbows and maximize diameter also facilitate future expansions. Similarly, using lower air speeds in air handlers often saves first costs and energy costs because less horsepower is required to perform the same task.
Increasing the cross-sectional area of the air handling unit (AHU) and using high performance filters adds to investment costs, but savings from smaller motors, fans and VFDs often reduce the overall air handling unit costs. It can also reduce electrical and emergency backup requirements and save space.
Recirculation fans used in low-pressure drop HVAC design strategies can provide substantial energy savings. Recirculation fans increase the air-change rates of filtered air, avoiding the added pressure drop of the air handler components. An associated air handler provides make up air for temperature and humidity control.
Further efficiencies can result from utilizing the space above the clean room ceiling as a distribution plenum instead of using ductwork to each filter.
Improved engineering also can reduce manufacturing facility shutdowns, leading to operational cost savings many times greater than the energy savings. For example, areas served by direct drive sealed bearing recirculation fans only need to be shut down every 4 to 8 years to change the HEPA filters, and can often remain in operation during HVAC system modifications.
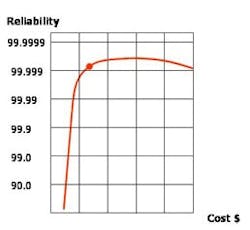
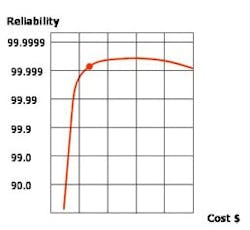
If no hazards or product contamination concerns would result, this air can be reused for less critical applications such as mechanical room ventilation. Heat recovery and other utility recycling can be beneficial, although any potential reliability concerns must be considered closely. Cost estimates for any mission-critical pharmaceutical manufacturing facility must consider reliability (see Graph, above) and the total cost of ownership and operation.
Because drug manufacturing is such an energy intensive business, energy-efficient mechanical/electrical systems can have a tremendous effect on the life cycle costs of the facility. Implementing the 3Rs of design redundancy, reliability and recovery is essential to achieve the best balance between cost and life cycle value.
About the Authors
Gary Shamshoian, P.E., is a Senior Mechanical Engineer, Genentech Inc., South San Francisco, Calif.
Don Nurisso, P.E., is a Senior Associate, EYP Mission Critical Facilities, San Francisco, Calif.
Tips for Enhancing Uptime and Minimizing Operating Costs
|